|
Spectator Ligand
In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such that the M-spectator ensemble is inert kinetically. Although they do not participate in reactions of the metal, spectator ligands influence the reactivity of the metal center to which they are bound. These ligands are sometimes referred to as ancillary ligands. Several different classes of ligand exist that can be considered spectator ligands. A few examples include trispyrazolylborates (Tp), cyclopentadienyl ligands (Cp), and many chelating diphosphines such as 1,2-bis(diphenylphosphino)ethane ligands (dppe). Varying the substituents on the spectator ligands greatly influences the solubility, stability, electronic, and steric properties of the metal complex. In the area of platinum-based antineoplastic Platinum-based antineoplastic drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polydentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be unidentate or monodentate. Ligands with more than one bonded atom are called multidentate or polydentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding sites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trispyrazolylborate
The trispyrazolylborate ligand, abbreviated Tp−, is an anionic Denticity, tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−. However, the term can also be used to refer to derivatives having substituents on the pyrazolyl rings. This class of compounds belongs to the family of ligands called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry group, symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin crossover, spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K[HB(C3N2H3)3] + 3H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Ligand
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Examples ''Bis''cyclopentadienyl complexes are called metallocenes. A famous example of this type of complex is ferrocene (FeCp2), which has many analogues for other metals, such as chromocene (CrCp2), cobaltocene (CoCp2), and nickelocene (NiCp2). When the Cp rings are mutually parallel the compound is known as a sandwich complex. This area of organometallic chemistry was first developed in the 1950s. Bent metallocenes are represented by compounds of the type Cp2Lx Some are catalysts for ethylene polymerization. Metallocenes are often thermally stable, and find use as cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
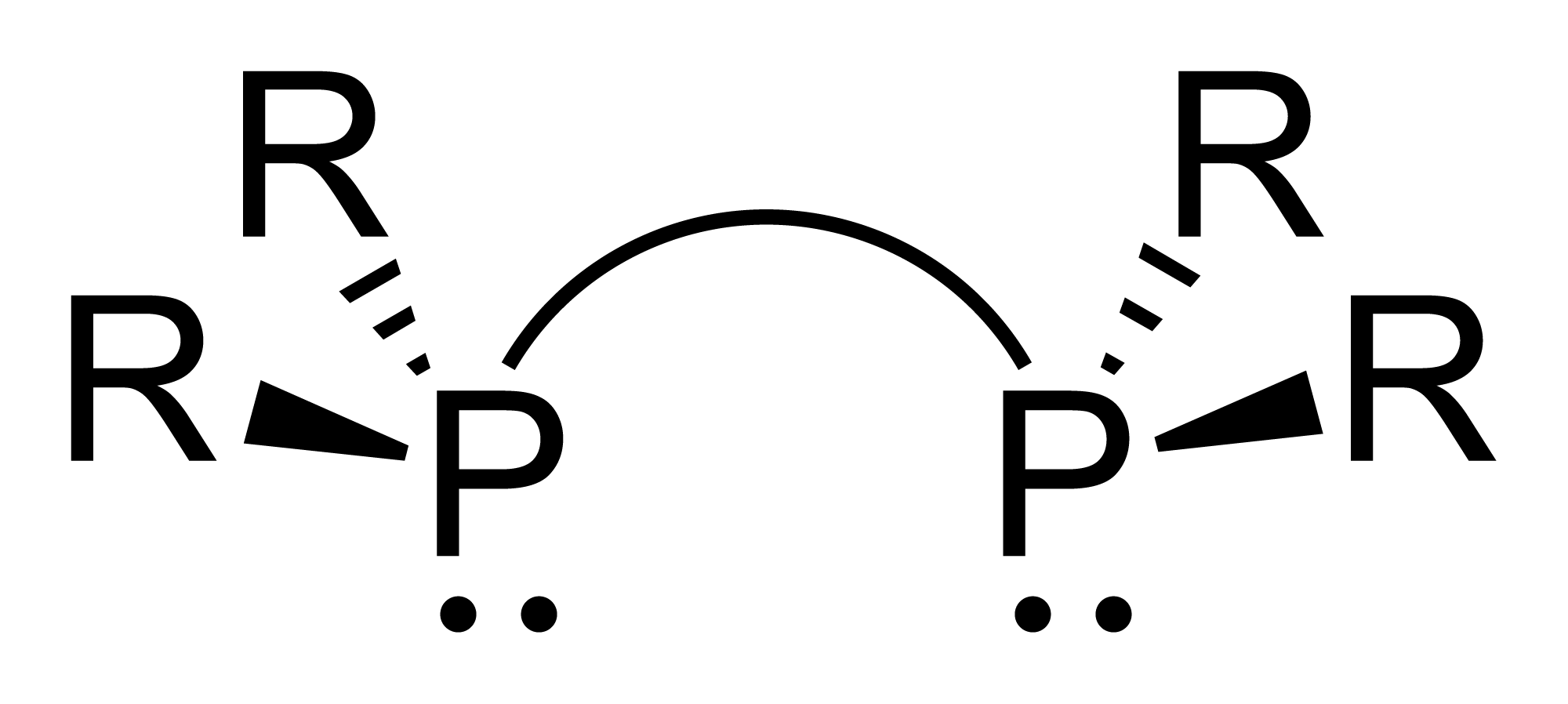
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents. Preparation The preparation of dppe entails the alkylation of NaP(C6H5)2 with 1,2-dichloroethane: : Reactions The reduction of dppe by lithium give the disecondary phosphine: : Hydrolysis gives the bis(secondary phosphine). : : Treatment of dppe with hydrogen peroxide produces the phosphine oxides .Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd Selective mono-oxidation of dppe can be achieved by benzylation followed by hydrolysis: : : Hydrogenation of dppe gives the ligand bis(dicyclohexylphosphino)ethane. Coordination complexes Many coordination complexes of dppe are known, and some are used as homogeneous catalysts. Dppe is almost invariably chelating, although there are examples of monodentate (e.g., W(CO)(d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum-based Antineoplastic
Platinum-based antineoplastic drugs (informally called platins) are chemotherapeutic agents used to treat cancer. Their active moieties are coordination complexes of platinum. These drugs are used to treat almost half of people receiving chemotherapy for cancer. In this form of chemotherapy, commonly used drugs include cisplatin, oxaliplatin, and carboplatin, but several have been proposed or are under development. Addition of platinum-based chemotherapy drugs to chemoradiation in women with early cervical cancer seems to improve survival and reduce risk of recurrence. In total, these drugs can cause a combination of more than 40 specific side effects which include neurotoxicity, which is manifested by peripheral neuropathies including polyneuropathy. Mechanism of action As studied mainly on cisplatin, but presumably for other members as well, platinum-based antineoplastic agents cause crosslinking of DNA as monoadduct, interstrand crosslinks, intrastrand crosslinks or DNA prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PdCl2(dppe)-3D-balls
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the chemical formula, formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures. Structure Two forms of PdCl2 are known, denoted α and β. In both forms, the palladium centres adopt a square-planar coordination geometry that is characteristic of Pd(II). Furthermore, in both forms, the Pd(II) centers are linked by μ2-chloride bridging ligand, bridges. The α-form of PdCl2 is a polymer, consisting of "infinite" slabs or chains. The β-form of PdCl2 is molecular, consisting of an octahedral cluster of six Pd atoms. Each of the twelve edges of this octahedron is spanned by Cl−. Platinum(II) chloride, PtCl2 adopts similar structures, whereas Nickel(II) chloride, NiCl2 adopts the Cadmium chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |