|
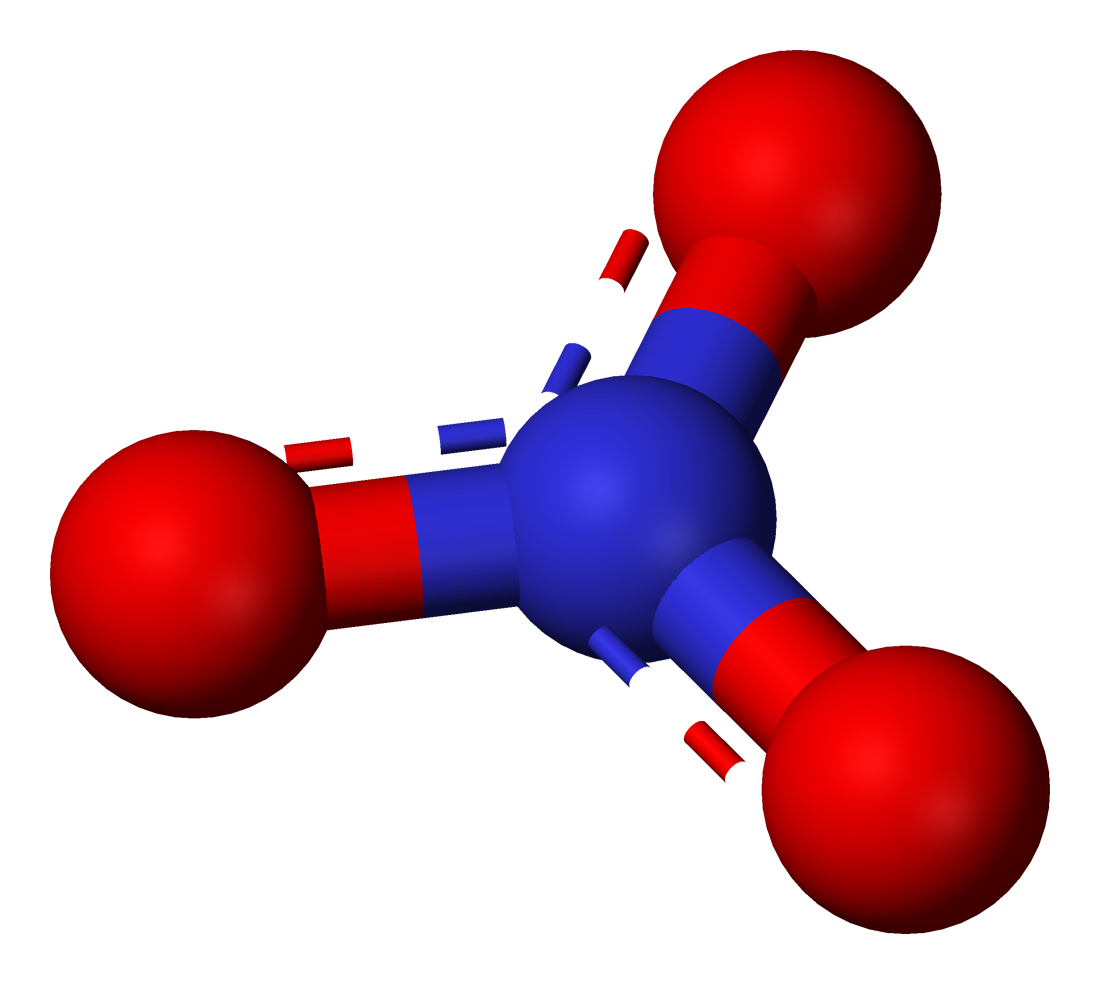
Sodium Nitrate
Sodium nitrate is the chemical compound with the chemical formula, formula . This alkali metal nitrate salt (chemistry), salt is also known as Chile saltpeter (large deposits of which were historically mined in Chile) to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter. Sodium nitrate is a white deliquescent solid very soluble in water. It is a readily available source of the nitrate anion (NO3−), which is useful in several reactions carried out on industrial scales for the production of fertilizers, pyrotechnics, smoke bombs and other explosives, glass and pottery vitreous enamel, enamels, food preservatives (esp. meats), and rocket propellant, solid rocket propellant. It has been mined extensively for these purposes. History The first shipment of saltpeter to Europe arrived in England from Peru in 1820 or 1825, right after that country's independence from Spain, but did not find any buyers and was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal Nitrate
Alkali metal nitrates are chemical compounds consisting of an alkali metal (lithium, sodium, potassium, rubidium and caesium) and the nitrate ion. Only two are of major commercial value, the sodium and potassium salts. They are white, water-soluble salts with melting points ranging from 255 °C () to 414 °C () on a relatively narrow span of 159 °C The melting point of the alkali metal nitrates tends to increase from 255 °C to 414 °C (with an anomaly for rubidium being not properly aligned in the series) as the atomic mass and the ionic radius (naked cation) of the alkaline metal increases, going down in the column. Similarly, but not presented here in the table, the solubility of these salts in water also decreases with the atomic mass of the metal. Applications Sodium and potassium nitrates are commonly used as fertilizers. As they are also strong oxidizers, they enter pyrotechnic compositions and the manufacturing of explosives. Eutectic mixtures of alkali metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be: * chemical energy, such as nitroglycerin or Dust explosion, grain dust * pressure, pressurized gas compressor, gas, such as a gas cylinder, aerosol can, or boiling liquid expanding vapor explosion * nuclear weapon, nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smoke Bomb
A smoke bomb is a firework designed to produce a large amount of smoke upon ignition. History Early Japanese history saw the use of a rudimentary form of the smoke bomb. Explosives were common in Japan during the Mongol invasions of the 13th century. Soft cased hand-held bombs were later designed to release smoke, poison gas, and shrapnel made from iron and pottery. The modern smoke bomb was created in 1848, by the British inventor Robert Yale. He developed 17th-century Chinese-style fireworks and later modified the formula to produce more smoke for a longer period. Colored smoke devices use a formula that consists of an oxidizer (typically potassium nitrate, KNO3), a fuel (generally sugar), a moderator (such as sodium bicarbonate) to keep the reaction from getting too hot, and a powdered organic dye. The burning of this mixture boils the dye and forces it out of the device, where it condenses in the atmosphere to form a smoke of finely dispersed particles. Home-made s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrotechnics
Pyrotechnics is the science and craft of creating fireworks, but also includes safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts (and other fasteners), parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. This trade relies upon self-contained and self-sustained exothermic chemical reactions to make heat, light, gas, smoke and/or sound. The name etymology, comes from the Greek words ''pyr'' (πυρ; 'fire') and ''technikós'' (τεχνικός; 'artistic'). Improper use of pyrotechnics could lead to List of pyrotechnic incidents, pyrotechnic accidents. People responsible for the safe storage, handling, and functioning of pyrotechnic devices are known as pyrotechnicians. Proximate pyrotechnics Explosions, flashes, smoke, flames, fireworks and other pyrotechnic-driven effects used in the entertainment industry are referred to as proximate pyrotechnics. Proximate refers to the pyrotechnic device's location relative to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods. Historically, fertilization came from natural or organic sources: compost, animal manure, human manure, harvested minerals, crop rotations, and byproducts of human-nature industries (e.g. fish processing waste, or bloodmeal from animal slaughter). However, starting in the 19th cen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they dissolve in the water they absorb, forming an aqueous solution. Hygroscopy is essential for many plant and animal species' attainment of hydration, nutrition, reproduction and/or seed dispersal. Biological evolution created hygroscopic solutions for water harvesting, filament tensile strength, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niter
Niter or nitre is the mineral form of potassium nitrate, KNO3. It is a soft, white, highly soluble mineral found primarily in arid climates or cave deposits. Potassium and other nitrates are of great importance for use in fertilizers and, historically, gunpowder. Much of the world's demand is now met by synthetically produced nitrates, though the natural mineral is still mined and is still of significant commercial value. Historically, the term ''niter'' was not well differentiated from natron, both of which have been very vaguely defined but generally refer to compounds of sodium or potassium joined with carbonate or nitrate ions. Characteristics Niter is a colorless to white mineral crystallizing in the orthorhombic crystal system. It is the mineral form of potassium nitrate, , and is soft (Mohs hardness 2), highly soluble in water, and easily fusible. Its crystal structure resembles that of aragonite, with potassium replacing calcium and nitrate replacing carbonate. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitratine
Nitratine or nitratite, also known as cubic niter (UK: nitre), soda niter or Chile saltpeter (UK: Chile saltpetre), is a mineral, the naturally occurring form of sodium nitrate, NaNO3. Chemically it is the sodium analogue of saltpeter. Nitratine crystallizes in the trigonal system, but rarely occurs as well-formed crystals. It is isostructural with calcite. It is relatively soft and light with a Mohs hardness of 1.5 to 2 and a specific gravity of 2.24 to 2.29. Its refractive indices are nω = 1.587 and nε = 1.336. The typical form is as coatings of white, grey to yellowish brown masses. The rare crystals when found typically have the scalenohedral form of the calcite structure. It is found only as an efflorescence in very dry environments. It is very soluble in water such that it is deliquescent and will absorb water out of the air and turn into a puddle of sodium nitrate solution when exposed to humid air. There are nitratine deposits located in arid regions across the world ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |