|
Silicate Esters
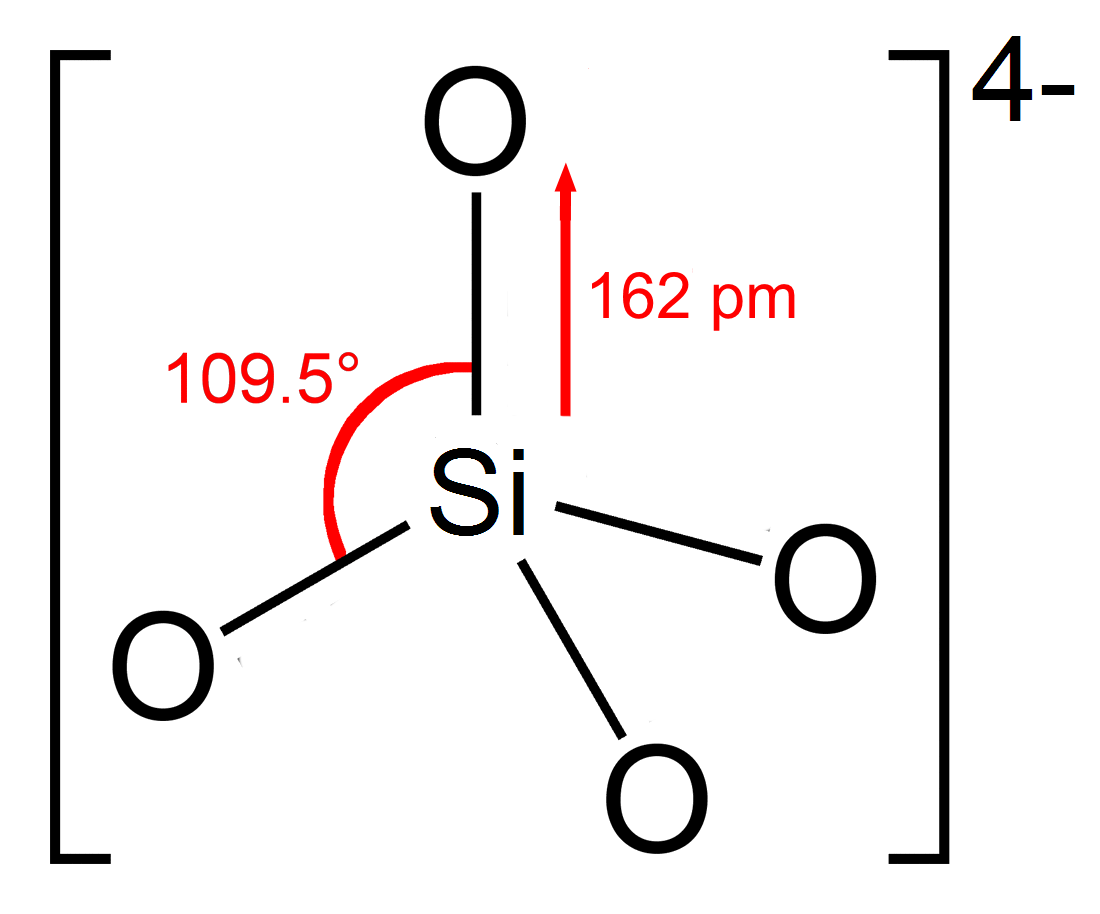
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate . Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In most silicates, a silicon atom occupies the center of an idealized tetrahedron whose corners are four o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portland Cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th century by Joseph Aspdin, and is usually made from limestone. It is a fine powder (substance), powder, produced by heating limestone and clay minerals in a kiln to form Clinker (cement), clinker, and then #Cement grinding, grinding the clinker with the addition of several percent (often around 5%) gypsum. Several types of portland cement are available. The most common, historically called ordinary portland cement (OPC), is grey, but white portland cement is also available. The cement was so named by Joseph Aspdin, who obtained a patent for it in 1824, because, once hardened, it resembled the fine, pale limestone known as Portland stone, quarried from the windswept cliffs of the Isle of Portland in Dorset. Portland stone was p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron (Fe(II)) or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron (Fe(II) or Fe(III)). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes. The name ''pyroxene'' is derived from the Ancient Greek w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, the crystalline forms of silica (silicon dioxide, ) are usually considered to be Silicate mineral#Tectosilicates, tectosilicates, and they are classified as such in the Dana system (75.1). However, the Nickel-Strunz system classifies them as oxide minerals (4.DA). Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomer, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass.'' The name is composed of Greek language, Greek elements ''wikt:oligo-, oligo-'', "a few" and ''wikt:-mer, -mer'', "parts". An adjective form is ''oligomeric''. The oligomer concept is contrasted to that of a polymer, which is usually understood to have a large number of units, possibly thousands or millions. However, there is no sharp distinction between these two concepts. One proposed criterion is whether the molecule's properties vary significantly with the removal of one or a few of the units. An oligomer with a specific number of units is referred t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Na2SiO3idealized
NA, N.A., Na, nA or n/a may refer to: Chemistry and physics * Sodium, symbol Na, a chemical element * Avogadro constant (''N''A) * Nucleophilic addition, a type of reaction in organic chemistry * Numerical aperture, a number that characterizes a range of angles in an optical system * nA, the symbol for nanoampere * Naturally aspirated engine Biology and medicine * Na (tree) or ''Mesua ferrea'', a species of tree native to Sri Lanka * Neuroacanthocytosis, a neurological condition * '' Nomina Anatomica'', a former international standard for human anatomical nomenclature * Noradrenaline, a hormone * Nucleic acid analogue, compounds analogous to naturally occurring RNA and DNA Places Current * Namibia (ISO country code) * Naples (car number plate code: NA), Italy * North America, a continent * North Africa, a subcontinent Historical * Netherlands Antilles (former international vehicle registration code: NA) * Na (Chinese state), a small state of the Chinese Zhou dynasty from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. Olivine has many uses, such as the gemstone peridot (or chrysolite), as well as industrial applications like metalworking processes. The ratio of magnesium to iron varies between the two endmember (mineralogy), endmembers of the solid solution series: forsterite (Mg-endmember: ) and fayalite (Fe-endmember: ). Compositions of olivine are commonly expressed as Mole (unit), molar percentages of forsterite (Fo) and/or fayalite (Fa) (''e.g.'', Fo70Fa30, or just Fo70 with Fa30 implied). Forsterite's melting temperature is unusually high at atmospheric pressure, almost , while fayalite's is much lower – about . Melting temperature varies smoothly between the two end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, and the 18-electron rule for transition metals. The valence electrons in molecules like carbon dioxide (CO₂) can be visualized using a Lewis electron dot diagram. In covalent bonds, electrons shared between two atoms are counted toward the octet of both atoms. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tetrahedron is the simplest of all the ordinary convex polytope, convex polyhedra. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean geometry, Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid (geometry), pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron, the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such net (polyhedron), nets. For any tetrahedron there exists a sphere (called th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |