|
Pterobranchia Mitochondrial Code
The pterobranchia mitochondrial code (translation table 24) is a genetic code used by the mitochondrial genome of ''Rhabdopleura compacta'' (Pterobranchia). The Pterobranchia are one of the two groups in the Hemichordata which together with the Echinodermata and Chordata form the three major lineages of deuterostomes. AUA translates to isoleucine in ''Rhabdopleura'' as it does in the Echinodermata and Enteropneusta while AUA encodes methionine in the Chordata. The assignment of AGG to lysine is not found elsewhere in deuterostome mitochondria but it occurs in some taxa of Arthropoda. This code shares with many other mitochondrial codes the reassignment of the UGA STOP to tryptophan, and AGG and AGA to an amino acid other than arginine. The initiation codons in ''Rhabdopleura compacta'' are ATG and GTG. Code 24 is very similar to the mitochondrial code 33 for the Pterobranchia. The code : AAs = FFLLSSSSYY**CCWWLLLLPPPPHHQQRRRRIIIMTTTTNNKKSSSKVVVVAAAADDEEG ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genetic Code
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries. The codons specify which amino acid will be added next during protein biosynthesis. With some exceptions, a three-nucleotide codon in a nucleic acid sequence specifies a single amino acid. The vast majority of genes are encoded with a single scheme (see the RNA codon table). That scheme is often referred to as the canonical or standard genetic code, or simply ''the'' genetic code, though variant codes (such as in mitochondria) exist. History Effo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cephalodiscidae Mitochondrial Code
The Cephalodiscidae mitochondrial code (translation table 33) is a genetic code used by the mitochondrial genome of Cephalodiscidae (Pterobranchia). The Pterobranchia are one of the two groups in the Hemichordata which together with the Echinodermata and Chordata form the major clades of deuterostomes. Code 33 is very similar to the mitochondrial code 24 for the Pterobranchia, which also belong to the Hemichordata, except that it uses UAA for tyrosine rather than as a stop codon. This code shares with many other mitochondrial codes the reassignment of the UGA STOP to tryptophan, and AGG and AGA to an amino acid other than arginine. However, the assignment of AGG to lysine in pterobranchian mitogenomes is not found elsewhere in deuterostome mitochondria but it occurs in some taxa of Arthropoda. The code : AAs = FFLLSSSSYYY*CCWWLLLLPPPPHHQQRRRRIIIMTTTTNNKKSSSKVVVVAAAADDEEGGGG : Starts = ---M-------*-------M---------------M---------------M------------ : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
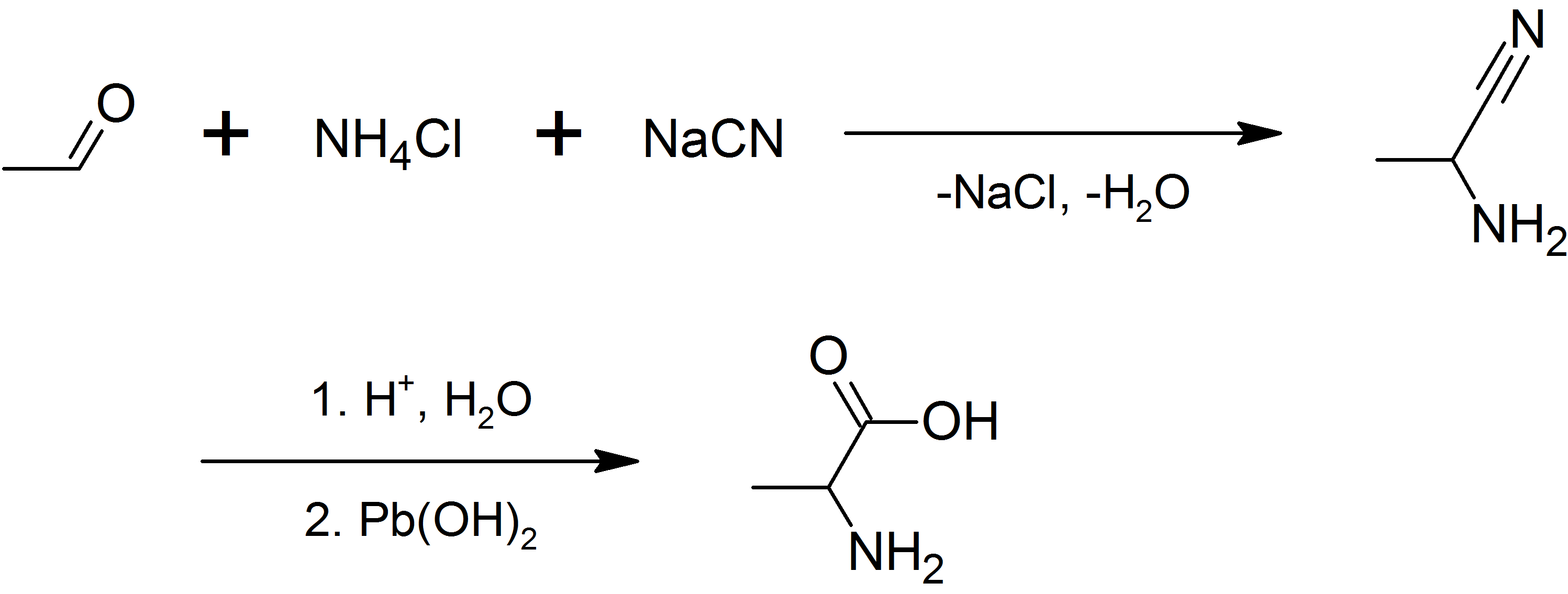
Glutamic Acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α- amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non- essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. ">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at the N-termini of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC. History Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant). It was isolated from asparagus juice, in which it is abundant, hence the chosen name. It was the first amino acid to be isolated. Three years later, in 1809, Pierre Jean Robiquet identified a substance from liquorice root with properties which he qualified as very similar to those of asparagine, and which Plisson i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine. Uracil is a common and naturally occurring pyrimidine derivative. The name "uracil" was coined in 1885 by the German chemist Robert Behrend, who was attempting to synthesize derivatives of uric acid. Originally discovered in 1900 by Alberto Ascoli, it was isolated by hydrolysis of yeast nuclein; it was also found in bovine thymus and spleen, herring sperm, and wheat germ. It is a planar, unsaturated compound that has the ability to absorb light. Based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, it is believed that uracil, xanthine, and related molecules can also be formed extraterrestrially. Data from the Cassini mission, orbitin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymine
Thymine () (symbol T or Thy) is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. In RNA, thymine is replaced by the nucleobase uracil. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calf thymus glands, hence its name. Derivation As its alternate name (5-methyluracil) suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thereby stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate), dTDP, or dTTP (for the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine
Guanine () (symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine- imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar. Properties Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form. It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine. In Watson-Crick base pairing, it forms three hydrogen bonds with guanine. History Cytosine was discovered and named by Albrecht Kossel and Albert Neumann in 1894 when it was hydrolyzed from calf thymus tissues. A structure was proposed in 1903, and was synthesized (and thus confirmed) in the laboratory in the same year. In 1998, cytosine was used in an early demonstration of quantum information processing when Oxford University researchers implemented the Deutsch-Jozsa algorithm on a two qubit nuclear magnetic resonance quantum computer (NMRQC). In March 2015, NASA scientists reported the formation of cytosine, along with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |