|
Polyimide
Polyimide (sometimes abbreviated PI) is a monomer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, such as high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids/diester. However, the first polyimide of significant co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset Polymer Matrix
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle. They were first used after World War II, and continuing research has led to an increased range of thermoset resins, polymers or plastics, as well as engineering grade thermoplastics. They were all developed for use in the manufacture of polymer composites with enhanced and longer-term service capabilities. Thermoset polymer matrix technologies also find use in a wide diversity of non-structural industrial applications. The foremost types of thermosetting polymers used in structural composites are benzoxazine resins, bis-maleimide resins (BMI), cyanate ester resins, epoxy (epoxide) resins, phenolic (PF) resins, unsaturated polyester ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kapton
file:Kaptonpads.jpg, Kapton insulating pads for mounting electronic parts on a heat sink Kapton is a polyimide film used in flexible printed circuits (flexible electronics) and space blankets, which are used on spacecraft, satellites, and various space instruments. Invented by the DuPont (1802-2017), DuPont Corporation in the 1960s, Kapton remains stable across a wide range of temperatures, from . Kapton is used in electronics manufacturing and space applications, with x-ray equipment, and in 3D printing applications. Its favorable thermal properties and outgassing characteristics result in its regular use in Cryogenics, cryogenic applications and in high vacuum environments. History Kapton was invented by DuPont in the 1960s. Kapton remains manufactured by DuPont to this day. The name ''Kapton'' is a registered trademark of E. I. du Pont de Nemours and Company. Chemistry and variants Kapton synthesis is an example of the use of a wikt:dianhydride, dianhydride in step pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called Polyimide, polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Examples Simple example is diacetamide with the formula , formally the diacetylated derivative of ammonia. Commonly encountered imides, however, are cyclic, being derived from dicarboxylic acids. A common example is succinimide derived from succinic acid and ammonia. The names of these cyclic imides reflect the parent acid. Many imides are derived from primary amines as opposed to ammonia. These are indicated by ''N''-substituent in the prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyromellitic Dianhydride
Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white, hygroscopic solid. It forms a hydrate. Preparation It is prepared by gas-phase oxidation of 1,2,4,5-tetramethylbenzene (or related tetrasubstituted benzene derivatives). An idealized equation is: :C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride. Reactions PMDA is an electron-acceptor, forming a variety of charge-transfer complexes. It reacts with amines to diimides, C6H2 CO)2NRsub>2 which also have acceptor properties. Applications PMDA is used in PET bottle recycling as a chain extender. It increases the molecular weight of the polymer by linking-together alcohol and carboxylic acid groups formed by hydrolysis of the PET. This improves the rheological properties and overall q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent (''catalyst'', '' hardener''). Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network. The starting material for making thermosets is usually malleable or liquid prior to curing, and is often designed to be molded into the final shape. It may also be used as an adhesive. Once hardened, a thermoset cannot be melted for reshaping, in contrast to thermoplastic polymers which are commonly produced and distributed in the form of pellets, and shaped into the final product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
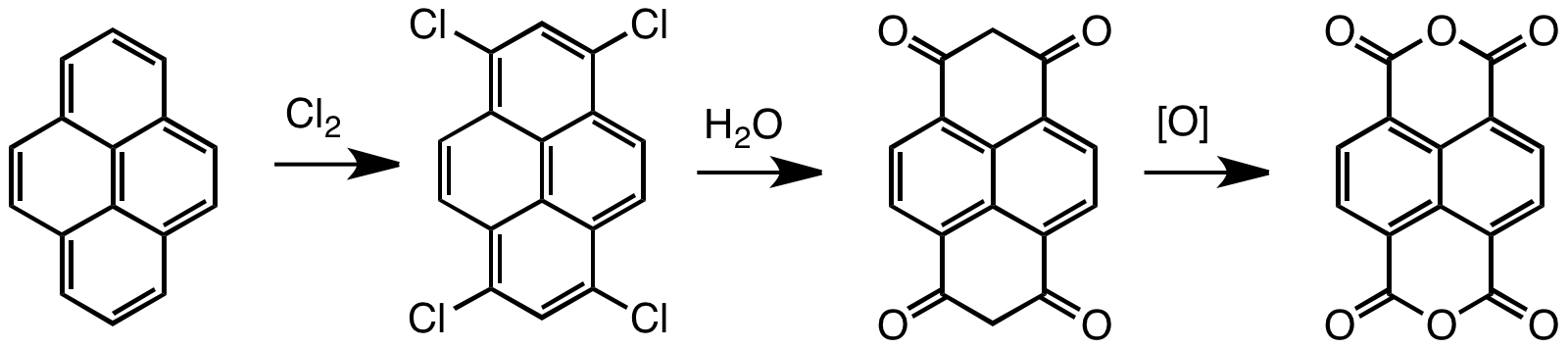
Naphthalene Tetracarboxylic Dianhydride
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs) (such as napthalenetetracarboxylic diimide), a family of compounds with many uses. Synthesis and structure Naphthalenetetracarboxylic dianhydride is prepared by oxidation of pyrene. Typical oxidants are chromic acid and chlorine. The unsaturated tetrachloride hydrolyzes to enols that tautomerize to the bis-dione, which in turn can be oxidized to the tetracarboxylic acid.F. Röhrscheid "Carboxylic Acids, Aromatic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Naphthalene diimides Symmetrical naphthalene diimides are synthesized by the condensation reaction of primary amines and the dianhydride. Unsymmetrical derivatives, i.e. those derived from two different amines, are obtained by hydrolysis of one of the two anhydride groups prior to the condensat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-diaminodiphenyl Ether
4,4′-Oxydianiline (ODA) is an organic compound with the chemical formula, formula Oxygen, O(Carbon, C6Hydrogen, H4Nitrogen, NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and Cross-link, cross-linking agent for polymers, especially the polyimides, such as Kapton. Uses 4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and Printed circuit board, printed circuits, and laminates and composite for aerospace vehicles. Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexural Strength
Flexural strength, also known as modulus of rupture, or bend strength, or transverse rupture strength is a material property, defined as the Stress (mechanics), stress in a material just before it Yield (engineering), yields in a flexure test. The transverse bending test is most frequently employed, in which a specimen having either a circular or rectangular cross-section is bent until fracture or yielding using a three-point flexural test technique. The flexural strength represents the highest stress experienced within the material at its moment of yield. It is measured in terms of stress, here given the symbol \sigma. Introduction When an object is formed of a single material, like a wooden beam or a steel rod, is bent (Fig. 1), it experiences a range of stresses across its depth (Fig. 2). At the edge of the object on the inside of the bend (concave face) the stress will be at its maximum compressive stress value. At the outside of the bend (convex face) the stress will b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |