|
Photothermal Optical Microscopy
Photothermal optical microscopy / "photothermal single particle microscopy" is a technique that is based on detection of non-fluorescent labels. It relies on absorption properties of labels ( gold nanoparticles, semiconductor nanocrystals, etc.), and can be realized on a conventional microscope using a resonant modulated heating beam, non-resonant probe beam and lock-in detection of photothermal signals from a single nanoparticle. It is the extension of the macroscopic photothermal spectroscopy to the nanoscopic domain. The high sensitivity and selectivity of photothermal microscopy allows even the detection of single molecules by their absorption. Similar to Fluorescence Correlation Spectroscopy (FCS), the photothermal signal may be recorded with respect to time to study the diffusion and advection characteristics of absorbing nanoparticles in a solution. This technique is called photothermal correlation spectroscopy (PhoCS). Forward detection scheme In this detection scheme a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
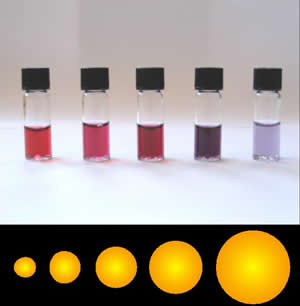
Colloidal Gold
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red (for spherical particles less than 100 nm) or blue-purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass, colloidal gold was used in the 4th-century Lycurgus Cup, which changes color dependi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Dot
Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic in nanotechnology and materials science. When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conduction band. The excited electron can drop back into the valence band releasing its energy as light. This light emission ( photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the discrete energy levels of the quantum dot in the conduction band and the valence band. In other words, a quantum dot can be defined as a structure on a semiconductor which is capable of confi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lock-in Amplifier
A lock-in amplifier is a type of amplifier that can extract a signal with a known carrier wave from an extremely noisy environment. Depending on the dynamic reserve of the instrument, signals up to a million times smaller than noise components, potentially fairly close by in frequency, can still be reliably detected. It is essentially a homodyne detection, homodyne detector followed by low-pass filter that is often adjustable in cut-off frequency and filter order. The device is often used to measure phase shift, even when the signals are large, have a high signal-to-noise ratio and do not need further improvement. Recovering signals at low signal-to-noise ratios requires a strong, clean reference signal with the same frequency as the received signal. This is not the case in many experiments, so the instrument can recover signals buried in the noise only in a limited set of circumstances. The lock-in amplifier is commonly believed to have been invented by Princeton University p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are distinguished from microparticles (1-1000 μm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the Brownian motion, they usually do not sediment, like colloid, colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nanoparticles cannot be seen with ordinary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Correlation Spectroscopy
Fluorescence correlation spectroscopy (FCS) is a statistical analysis, via time correlation, of stationary fluctuations of the fluorescence intensity. Its theoretical underpinning originated from L. Onsager's regression hypothesis. The analysis provides kinetic parameters of the physical processes underlying the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent particles (molecules) in solution. In this application, the fluorescence emitted from a very tiny space in solution containing a small number of fluorescent particles (molecules) is observed. The fluorescence intensity is fluctuating due to Brownian motion of the particles. In other words, the number of the particles in the sub-space defined by the optical system is randomly changing around the average number. The analysis gives the average number of fluorescent particles and average diffusion time, when the particle is passing through the space. Eventu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichroic Filter
In optics, a dichroic material is either one which causes visible light to be split up into distinct beams of different wavelengths (colours) (not to be confused with dispersion), or one in which light rays having different polarizations are absorbed by different amounts. In beam splitters The original meaning of ''dichroic'', from the Greek ''dikhroos'', two-coloured, refers to any optical device which can split a beam of light into two beams with differing wavelengths. Such devices include mirrors and filters, usually treated with optical coatings, which are designed to reflect light over a certain range of wavelengths and transmit light which is outside that range. An example is the dichroic prism, used in some camcorders, which uses several coatings to split light into red, green and blue components for recording on separate CCD arrays, however it is now more common to have a Bayer filter to filter individual pixels on a single CCD array. This kind of dichroic device doe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (electromagnetic Radiation)
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy—and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect of the absorption of electromagnetic radiation is attenuation of the radiation; attenuation is the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials such as mineral wool or Styrofoam. Metals have this high thermal conductivity due to free electrons facilitating heat transfer. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |