|
Photoelectric Effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoelectric Effect In A Solid - Diagram
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, Solid-state chemistry, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in Electronics, electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity (physics), intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile (can be drawn into a wire) and malleable (can be shaped via hammering or pressing). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polythiazyl, polymeric sulfur nitride. The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics are nonmetallic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compton Scattering
Compton scattering (or the Compton effect) is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound electrons from the outer valence shells of atoms or molecules. The effect was discovered in 1923 by Arthur Holly Compton while researching the scattering of X-rays by light elements, and earned him the Nobel Prize in Physics in 1927. The Compton effect significantly deviated from dominating classical theories, using both special relativity and quantum mechanics to explain the interaction between high frequency photons and charged particles. Photons can interact with matter at the atomic level (e.g. photoelectric effect and Rayleigh scattering), at the nucleus, or with just an electron. Pair production and the Compton effect occur at the level of the electron. When a high frequency photon scatters due to an interaction with a charged part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irradiated
Irradiation is the process by which an object is exposed to radiation. An irradiator is a device used to expose an object to radiation, most often gamma radiation, for a variety of purposes. Irradiators may be used for sterilizing medical and pharmaceutical supplies, preserving foodstuffs, alteration of gemstone colors, studying radiation effects, eradicating insects through sterile male release programs, or calibrating thermoluminescent dosimeters (TLDs). The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to normal levels of background radiation. The term irradiation usually excludes the exposure to non-ionizing radiation, such as infrared, visible light, microwaves from cellular phones or electromagnetic waves emitted by radio and television receivers and power supplies. Applications Sterilization If ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Binding Energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as :X(g) + energy ⟶ X+(g) + e− where X is any atom or molecule, X+ is the resultant ion when the original atom was stripped of a single electron, and e− is the removed electron. Ionization energy is positive for neutral atoms, meaning that the ionization is an endothermic process. Roughly speaking, the closer the outermost electrons are to the nucleus of the atom, the higher the atom's ionization energy. In physics, ionization energy (IE) is usually expressed in electronvolts (eV) or joules (J). In chemistry, it is expressed as the energy to ionize a mole of atoms or molecules, usually as kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Comparison of ionization energies of atoms in the periodic table reveals two periodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon Energy
Photon energy is the energy carried by a single photon. The amount of energy is directly proportional to the photon's electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength. The higher the photon's frequency, the higher its energy. Equivalently, the longer the photon's wavelength, the lower its energy. Photon energy can be expressed using any energy unit. Among the units commonly used to denote photon energy are the electronvolt (eV) and the joule (as well as its multiples, such as the microjoule). As one joule equals , the larger units may be more useful in denoting the energy of photons with higher frequency and higher energy, such as gamma rays, as opposed to lower energy photons as in the optical and radio frequency regions of the electromagnetic spectrum. Formulas Physics Photon energy is directly proportional to frequency. E = hf where * E is energy ( joules in the SI system) * h is the Planck constant * f is frequency T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
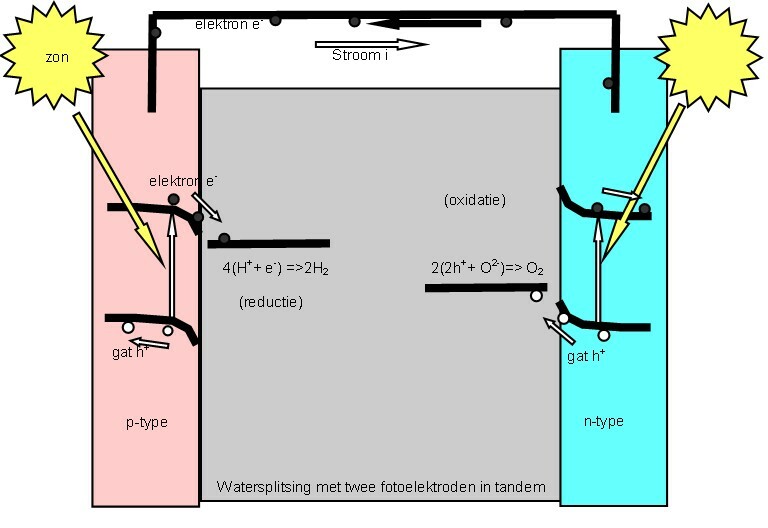
Photoelectrochemical Cell
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water. Both types of device are varieties of solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen). Two principles The standard photovoltaic effect, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaic Effect
The photovoltaic effect is the generation of voltage and electric current in a material upon exposure to light. It is a physical phenomenon. The photovoltaic effect is closely related to the photoelectric effect. For both phenomena, light is absorbed, causing excitation of an electron or other charge carrier to a higher-energy state. The main distinction is that the term ''photoelectric effect'' is now usually used when the electron is ejected out of the material (usually into a vacuum) and ''photovoltaic effect'' used when the excited charge carrier is still contained within the material. In either case, an electric potential (or voltage) is produced by the separation of charges, and the light has to have a sufficient energy to overcome the potential barrier for excitation. The physical essence of the difference is usually that photoelectric emission separates the charges by ballistic conduction and photovoltaic emission separates them by diffusion, but some "hot carrier" phot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoconductivity
Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation. When light is absorbed by a material such as a semiconductor, the number of free electrons and holes increases, resulting in increased electrical conductivity. To cause excitation, the light that strikes the semiconductor must have enough energy to raise electrons across the band gap, or to excite the impurities within the band gap. When a bias voltage and a load resistor are used in series with the semiconductor, a voltage drop across the load resistors can be measured when the change in electrical conductivity of the material varies the current through the circuit. Classic examples of photoconductive materials include: * photographic film: Kodachrome, Fujifilm, Agfachrome, Ilford, ''etc.'', based on silver sulfide and silver bromi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saunders (imprint)
Saunders is an American academic publisher based in the United States. It is currently an imprint of Elsevier. Formerly independent, the W. B. Saunders company was acquired by CBS in 1968, who added it to their publishing division Holt, Rinehart & Winston. When CBS left the publishing field in 1986, it sold the academic publishing units to Harcourt Brace Jovanovich. Harcourt was acquired by Reed Elsevier in 2001. . Northern Illinois University Libraries. Retrieved May 2, 2015. W. B. Saunders published the Kinsey Reports
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave–particle Duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the inability of the Classical physics, classical concepts such as particle or wave to fully describe the behavior of quantum objects. During the 19th and early 20th centuries, light was found to behave as a wave then later was discovered to have a particle-like behavior, whereas electrons behaved like particles in early experiments then were later discovered to have wave-like behavior. The concept of duality arose to name these seeming contradictions. History Wave-particle duality of light In the late 17th century, Sir Isaac Newton had advocated that light was Corpuscular theory of light, corpuscular (particulate), but Christiaan Huygens took an opposing wave description. While Newton had favored a particle approach, he was the first to at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom which contains protons, neutrons and electrons, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in daltons (making a quantity called the " relative isotopic mass"), is within 1% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |