|
PH Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamin
Dynamin is a GTPase protein responsible for endocytosis in the eukaryotic cell. Dynamin is part of the "dynamin superfamily", which includes classical dynamins, dynamin-like proteins, MX1, Mx proteins, OPA1, MFN1, mitofusins, and Guanylate-binding protein, GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicle (biology), vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface (particularly caveolae internalization) as well as at the Golgi apparatus.Hinshaw, J"Research statement, Jenny E. Hinshaw, Ph.D." National Institute of Diabetes & Digestive & Kidney Diseases, Laboratory of Cell Biochemistry and Biology. Accessed 19 March 2013. Dynamin family members also play a role in many processes including division of organelles, cytokinesis and microbial pathogen resistance. Structure Dynamin itself is a 96 kilodalton, kDa enzyme, and was first isolated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small G-protein
Small GTPases (), also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases. A typical G-protein is active when bound to GTP and inactive when bound to GDP (i.e. when the GTP is hydrolyzed to GDP). The GDP can then be replaced by free GTP. Therefore, a G-protein can be switched on and off. GTP hydrolysis is accelerated by GTPase activating proteins (GAPs), while GTP exchange is catalyzed by guanine nucleotide exchange factors (GEFs). Activation of a GEF typically activates its cognate G-protein, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IRS-1
Insulin receptor substrate 1 (IRS-1) is a signaling adapter protein that in humans is encoded by the ''IRS1'' gene. It is a 180 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-terminus and a PTB domain ca. 40 residues downstream of this, followed by a poorly conserved C-terminus tail. Together with IRS2, IRS3 (pseudogene) and IRS4, it is homologous to the ''Drosophila'' protein ''chico'', whose disruption extends the median lifespan of flies up to 48%. Similarly, Irs1 mutant mice experience moderate life extension and delayed age-related pathologies. Function Insulin receptor substrate 1 plays a key role in transmitting signals from the insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF-1) to intracellular pathways PI3K / Akt and Erk MAP kinase pathways. Tyrosine phosphorylation of IRS-1 by insulin receptor (IR) introduces multiple binding sites for proteins bearing SH2 homology domain, su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-receptor Tyrosine Kinase
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as Adenosine triphosphate, ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell. Unlike the receptor tyrosine kinases (RTKs), the second subgroup of tyrosine kinases, the non-receptor tyrosine kinases are cytosolic enzymes. Thirty-two non-receptor tyrosine kinases have been identified in human cells (). Non-receptor tyrosine kinases regulate cell growth, proliferation, differentiation, adhesion, migration and apoptosis, and they are critical components in the regulation of the immune system. Function The main function of nRTKs is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase D1
Serine/threonine-protein kinase D1 is an enzyme that in humans is encoded by the ''PRKD1'' gene. Function Members of the protein kinase D (PKD) family function in many extracellular receptor-mediated signal transduction pathways. The PRKCM gene encodes a cytosolic serine-threonine kinase that binds to the trans-Golgi network and regulates the fission of transport carriers specifically destined to the cell surface. upplied by OMIMref name="entrez" /> Interactions Protein kinase D1 has been shown to interact with: * Bruton's tyrosine kinase Bruton's tyrosine kinase (abbreviated Btk or BTK), also known as tyrosine-protein kinase BTK, is a tyrosine kinase that is encoded by the ''BTK'' gene in humans. BTK plays a crucial role in B cell development. Structure BTK contains five di ..., * C1QBP, * Centaurin, alpha 1, * Metallothionein 2A, and * YWHAQ. References Further reading * * * * * * * * * * * * * * * * * * * EC 2.7.11 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine/threonine-specific Protein Kinase
A serine/threonine protein kinase () is a kinase enzyme, in particular a protein kinase, that phosphorylation, phosphorylates the hydroxyl, OH group of the amino acid, amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK). In enzymology, the term ''serine/threonine protein kinase'' describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important post-translational modification. The chemical reaction performed by these enzymes can be written as :ATP + a protein \rightleftharpoons ADP + a phosphoprotein Thus, the two substrate (biochemistry), substrates of this enzyme are adenosine triphosphate, ATP and a protein, whereas its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin
Pleckstrins are a family of proteins found in platelets and other cells. The name derives from platelet and leukocyte C kinase substrate and the KSTR string of amino acids. The prototype protein, now called pleckstrin-1, was first identified in 1979 as the major substrate of protein kinase C in platelets. The homolog pleckstrin-2 is more widely expressed in tissues. The pleckstrin homology domain (PH domain) was named after pleckstrin-1. Sequence and structure Both pleckstrin-1 and pleckstrin-2 contain two pleckstrin homology domains, separated by a central dishevelled-Egl10-pleckstrin (DEP) domain. Pleckstrin-1 is phosphorylated by protein kinase C on three serine and threonine residues located between the first pleckstrin homology domain and the DEP domain; pleckstrin-2 is not a substrate for protein kinase C. The two proteins share 65% sequence homology and have a size of about 47 kilodaltons. As of 2024, no high-resolution three-dimensional structure has been solved f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharomyces Cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungal microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like '' Escherichia coli'' as the model bacterium. It is the microorganism which causes many common types of fermentation. ''S. cerevisiae'' cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding. Many proteins important in human biology were first discovered by studying their homologs in yeast; these proteins include cell cycle proteins, signaling proteins, and protein-processing enzymes. ''S. cerevisiae'' is currently the only yeast cell known to have Berkeley bodies present, which are involved in particular secretory pathways. Antibodies again ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
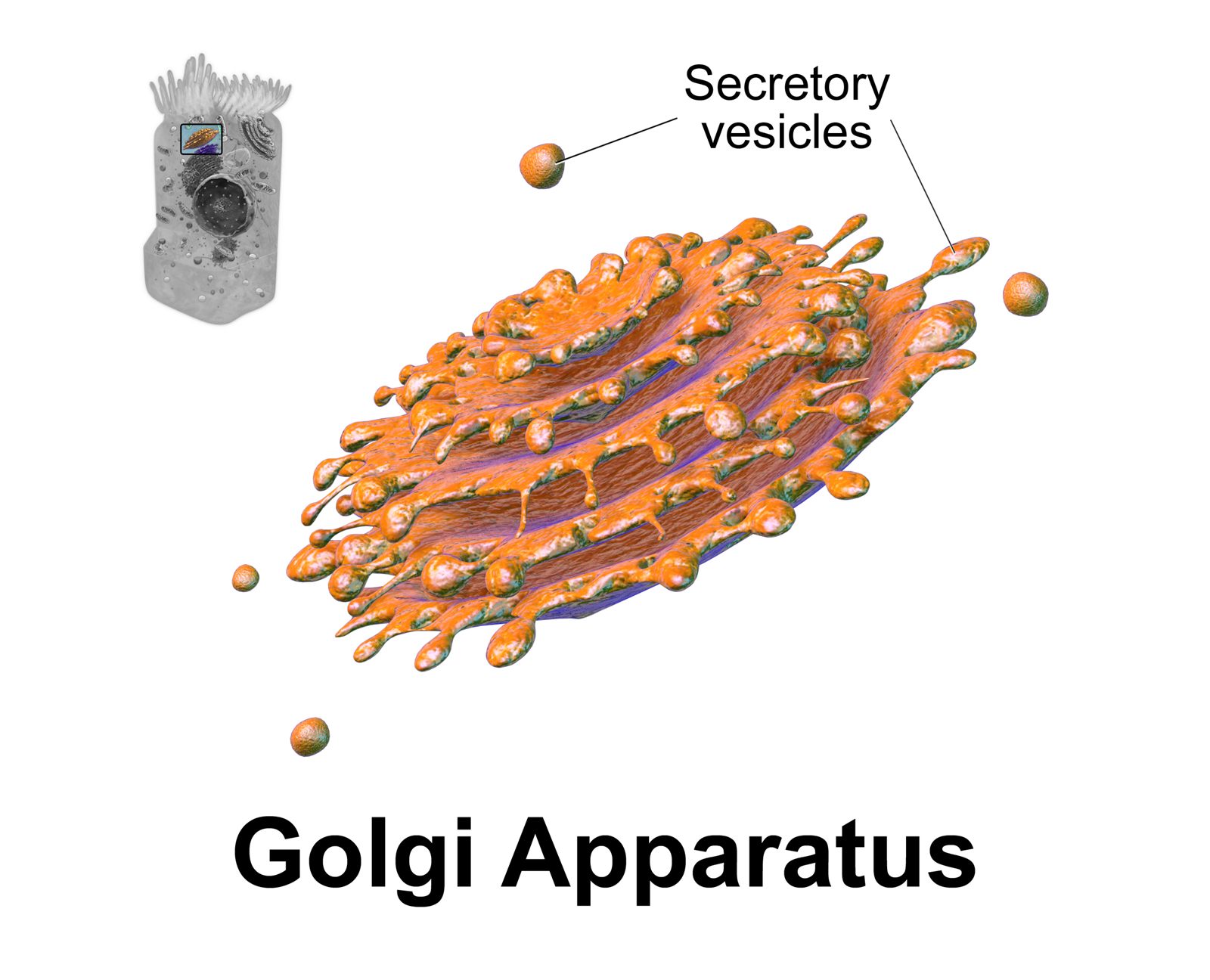
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins into membrane-bound Vesicle (biology and chemistry), vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and Endocytosis, endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. The Golgi apparatus was identified in 1898 by the Italian biologist and pathologist Camillo Golgi. The organelle was later named after him in the 1910s. Discovery Because of its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF (G-protein)
ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling. The small ADP ribosylation factor (Arf) GTP-binding proteins are major regulators of vesicle biogenesis in intracellular traffic. They are the founding members of a growing family that includes Arl (Arf-like), Arp (Arf-related proteins) and the remotely related Sar (Secretion-associated and Ras-related) proteins. Arf proteins cycle between inactive GDP-bound and active GTP-bound forms that bind selectively to effectors. The classical structural GDP/GTP switch is characterised by conformational changes at the so-called switch 1 and switch 2 regions, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |