 |
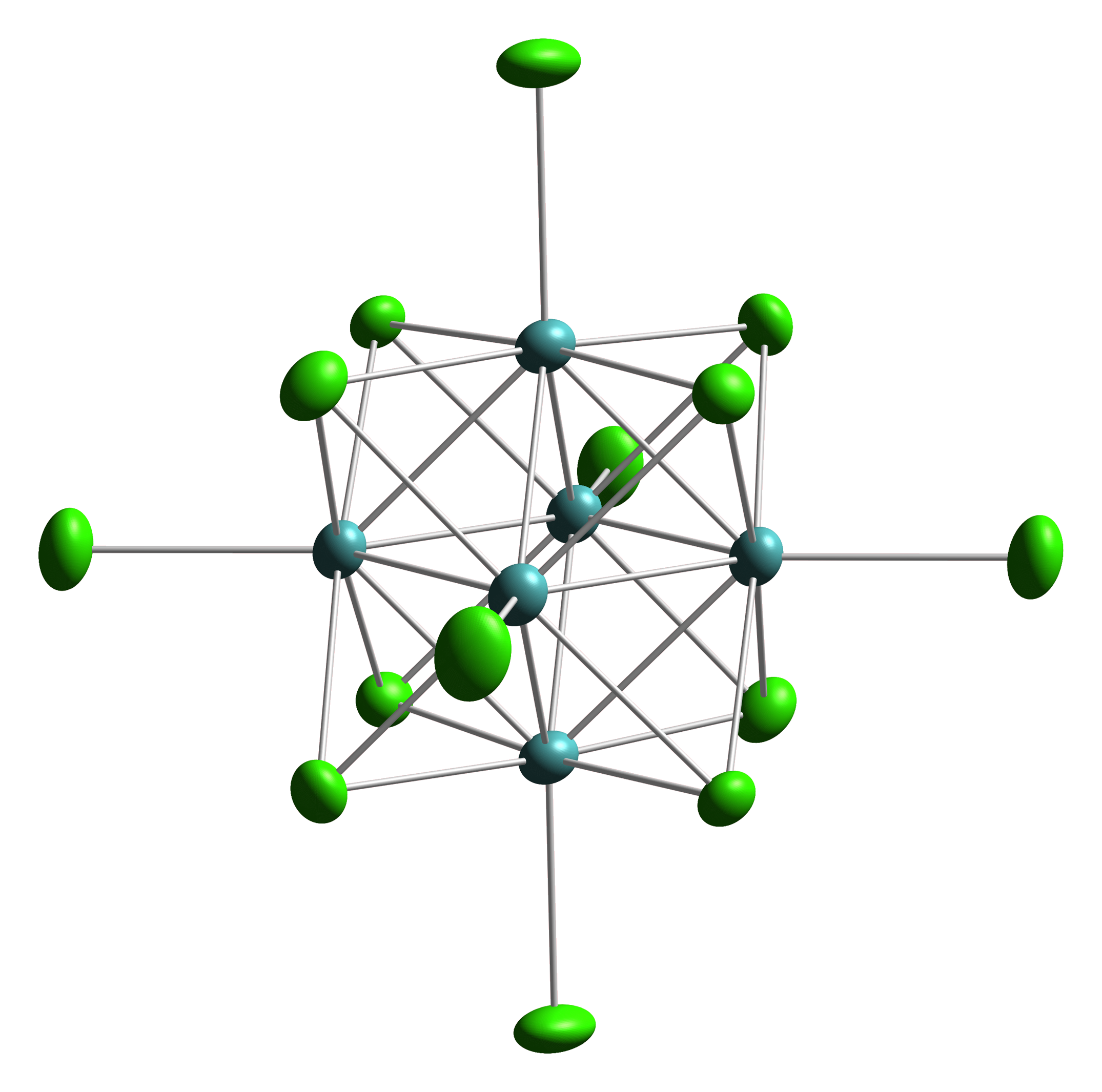
Octahedral Cluster
Octahedral clusters are inorganic or organometallic cluster (chemistry), cluster compounds composed of six metals in an octahedral molecular geometry, octahedral array.Eric J. Welch and Jeffrey R. Long ''Atomlike Building Units of Adjustable Character: Solid-State and Solution Routes to Manipulating Hexanuclear Transition Metal Chalcohalide Clusters'' in Progress in Inorganic Chemistry, Volume 54 Kenneth D. Karlin 2005''Link/ref> Many types of compounds are known, but all are synthetic. Octahedral chalcogenide and halide clusters These compounds are bound together by metal-metal bonding as well as two kinds of ligands. Ligands that span the faces or edges of the M6 core are labeled Li, for ''inner'' (innen in the original German description), and those ligands attached only to one metal are labeled outer, or La for ''ausser''. Typically, the outer ligands can be exchanged whereas the bridging ligands are more inert toward substitution. Face-capped halide clusters The premier e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Cluster (chemistry)
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. Materials can be categorized into three different regimes, namely bulk, nanoparticles and nanoclusters. Bulk metals are electrical conductors and good optical reflectors and metal nanoparticles display intense colors due to surface plasmon resonance. However, when the size of metal nanoclusters is further reduced to form a nanocluster, the band structure becomes discontinuous and breaks down into discrete energy levels, somewhat similar to the energy levels of molecules. This gives nanoclusters similar qu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a Native metal, free metal on Earth; in its minerals, it is found only in oxidation state, oxidized states. The free element, a silvery metal with a grey cast, has the List of elements by melting point, sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Iron Pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis. Properties Iron pentacarbonyl is a homoleptic metal carbonyl, where carbon monoxide is the only ligand complexed with a metal. Other examples include octahedral chromium hexacarbonyl (Cr(CO)6) and tetrahedral nickel carbonyl (Ni(CO)4). Most metal carbonyls have 18 valence electrons, and Fe(CO)5 fits this pattern with 8 valence electrons on Fe and five pairs of electrons provided by the CO ligands. Reflecting its symmetrical structure and charge neutrality, Fe(CO)5 is volatile; it is one of the most frequently encountered liquid metal complexes. Fe(CO)5 adopts a trigonal bipyramidal structure with the Fe atom surrounded by five CO ligands: thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Metal Carbonyl Cluster
In chemistry, a metal carbonyl cluster is a compound that contains two or more metal atoms linked in part by metal–metal bonds and containing carbon monoxide (CO) as the exclusive or predominant ligand. The area is a subfield of metal carbonyl chemistry, and many metal carbonyl clusters are in fact prepared from simple metal carbonyls. Simple examples include Fe2(CO)9, Fe3(CO)12, and Mn2(CO)10. High nuclearity clusters include h13(CO)24H3sup>2− and the stacked Pt3 triangules t3n(CO)6nsup>2− (n = 2–6). History The first metal carbonyl clusters, Fe3(CO)12, Ir4(CO)12, and Rh6(CO)16, were reported starting in the 1930s, often by Walter Hieber. The structures were subsequently established by X-ray crystallography. Paolo Chini (1928–1980) was a pioneer for the synthesis and characterization of high-nuclearity metal carbonyl clusters. His first studies started in 1958, in the attempt to repeat a patent that claimed an improved selectivity in hydroformylation. From a mixtu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
 |
Naked Cluster
Nudity is the state of being in which a human is without clothing. While estimates vary, for the first 90,000 years of pre-history, anatomically modern humans were naked, having lost their body hair, living in hospitable climates, and not having developed the crafts needed to make clothing. As humans became behaviorally modern, body adornments such as jewelry, tattoos, body paint and scarification became part of non-verbal communications, indicating a person's social and individual characteristics. Indigenous peoples in warm climates used clothing for decorative, symbolic or ceremonial purposes but were often nude, having neither the need to protect the body from the elements nor any conception of nakedness being shameful. In many societies, both ancient and contemporary, children might be naked until the beginning of puberty. Women may not cover their breasts due to the association with nursing babies more than with sexuality. In the ancient civilizations of the Medite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Wurtzite Crystal Structure
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais lat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Zincblende (crystal Structure)
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.excerpt Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Tungsten(III) Chloride
Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides. A related mixed valence W(III)-W(IV) chloride is prepared by reduction of the hexachloride with bismuth Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...: :9 WCl6 + 8 Bi → 3 W3Cl10 + 8 BiCl3 References {{Chlorides Tungsten halides Chlorides Octahedral compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Superconduction
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered, even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source. The superconductivity phenomenon was discovered in 1911 by Dutch physicist Heike Kamerlingh Onnes. Like ferromagnetism and atomic spectral lines, superconductivity is a phenomenon which can only be explained by quantum mechanics. It is characterized by the Meissner effect, the complete cancellation of the magnetic field in the interior of the superconductor during its transitions into the superconducting state. The occurrence of the Meissner effect indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elemental state or as pure ore compounds in Earth's crust. Selenium ( ) was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in :Sulfide minerals, metal sulfide ores, where it substitutes for sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores. Minerals that are pure selenide or selenate compounds are rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been mostly replaced with silicon semiconductor devices. Selenium is still used in a few types of Direct current, DC power surge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |