|
Noyori Asymmetric Hydrogenation
In chemistry, the Noyori asymmetric hydrogenation refers to methodology for enantioselective reduction of ketones and related functional groups. This methodology was introduced by Ryoji Noyori, who shared the Nobel Prize in Chemistry in 2001 for contributions to asymmetric hydrogenation. These hydrogenations are used in the production of several drugs, such as the antibacterial levofloxin, the antibiotic carbapenem, and the antipsychotic agent BMS181100. History The stoichiometric asymmetric reduction of ketones has long been known, e.g., using boron hydrides. The catalytic asymmetric hydrogenation of ketones was demonstrated with catalysts based on BINAP-Ru halides and carboxylates. Even though the BINAP-Ru dihalide catalyst could reduce functionalized ketones, the hydrogenation of simple ketones remained an unsolved. This challenge was solved with precatalysts of the type RuCl2(diphosphane)(diamine). These catalysts preferentially reduce ketones and aldehydes, leaving olefin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
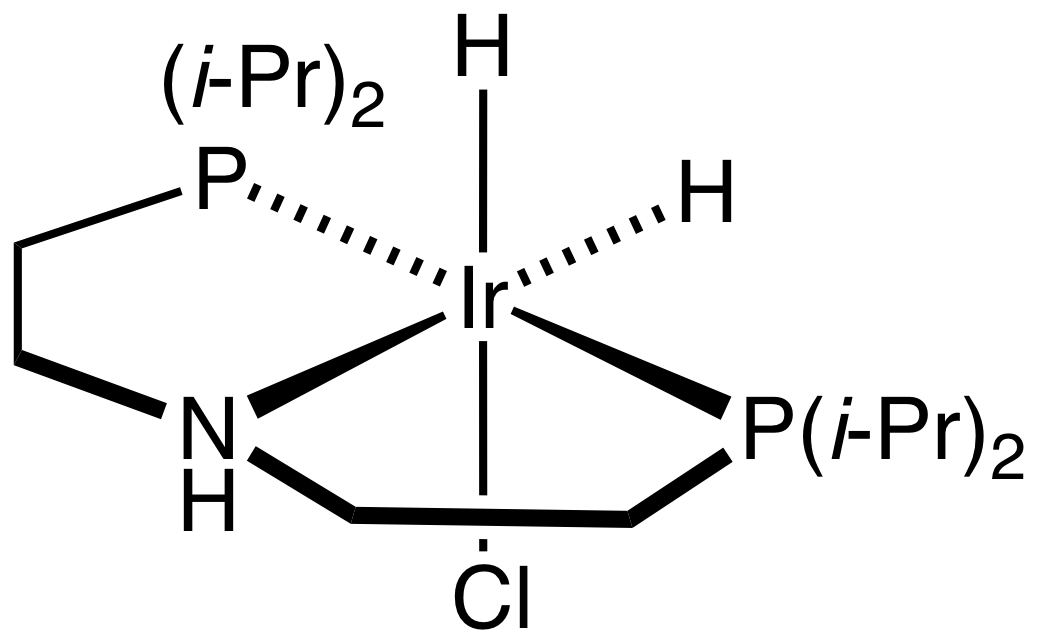
MACHO Catalyst
In homogeneous catalysis, MACHO catalysts are metal complexes containing MACHO ligands, which are of the type HN(CH2CH2PR2)2, where R is typically phenyl or isopropyl. Complexes with ruthenium(II) and iridium(III) have received much attention for their ability to hydrogenate polar bonds such as those in esters and even carbon dioxide. The catalysts appear to operate via intermediates where the amine proton and the hydride ligand both interact with the substrate. The Ru-MACHO catalyst have been commercialized for the synthesis of 1,2-propanediol from bio-derived methyl lactate.{{cite journal , doi=10.1038/s41570-018-0049-z, title=The role of the metal-bound N–H functionality in Noyori-type molecular catalysts, year=2018, last1=Dub, first1=Pavel A., last2=Gordon, first2=John C., s2cid=106394152, journal=Nature Reviews Chemistry, volume=2, issue=12, pages=396–408 See also * 1,5-Diaza-3,7-diphosphacyclooctanes, phosphine amine ligands used in hydrogen evolution * Noyori asymmetri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Kinetic Resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. The enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in Reactivity (chemistry), reactivity between enantiomers or enantiomeric complexes. Kinetic resolution can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbapenem
Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta lactam class of antibiotics, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams. Carbapenem antibiotics were originally developed at Merck & Co. from the carbapenem thienamycin, a naturally derived product of ''Streptomyces cattleya''. Concern has arisen in recent years over increasing rates of resistance to carbapenems, as there are few therapeutic options for treatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Lactate
Methyl lactate, also known as lactic acid methyl ester, is the organic compound with the formula CH3CH(OH)CO2CH3. It is the methyl ester of lactic acid. A colorless liquid, it is the simplest chiral ester. Being naturally derived, it is readily available as a single enantiomer. Uses It is a solvent for nitrocellulose, cellulose acetate, cellulose acetobutyrate and cellulose acetopropionate. It is used in the manufacture of lacquers and dopes where it contributes high tolerance for diluents, good flaw and blush resistance."Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. , The synthesis of 1,2-propanediol from methyl lactate has been commercialized using a MACHO catalyst. See also *Ethyl lactate Ethyl lactate, also known as lactic acid ethyl ester, is the organic compound with the formula CH3CH(OH)CO2CH2CH3. It is the ethyl ester of lactic acid. A colorless liquid, it is a chiral ester. Being naturally derived, it is readily availa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levofloxacin
Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori (in combination with other medications), urinary tract infections, chronic prostatitis, and some types of gastroenteritis. Along with other antibiotics it may be used to treat tuberculosis, meningitis, or pelvic inflammatory disease. Use is generally recommended only when other options are not available. It is available by mouth, intravenously, and in eye drop form. Common side effects include nausea, diarrhea, and trouble sleeping. Serious side effects may include tendon rupture, tendon inflammation, seizures, psychosis, and potentially permanent peripheral nerve damage. Tendon damage may appear months after treatment is completed. People may also sunburn more easily. In people with myasthenia gravis, muscle weakness and breathing problems may worsen. While use during pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |