|
Non-biological Complex Drugs
{{Short description, Medicines that are not manufactured using biotechnology Non-biological Complex Drugs (NBCDs) are medical compounds that cannot be defined as small molecular, fully identifiable drugs with active pharmaceutical ingredients. They are highly complex and cannot be defined as biologicals as they are not derived from living materials. NBCDs are synthetic complex compounds and they contain non-homomolecular, closely related molecular structures with often nanoparticular properties. This is, for instance, the case with the iron sucrose and its similars. But also with other drug products, e.g. polypeptides (glatiramoids), swelling polymers, liposomes as the NBCD class is growing.Crommelin, D. J., & de Vlieger, J. S. (2015). Epilogue: What Did We Learn? What Can We Expect in the Future? Concluding Remarks and Outstanding Issues. In ''Non-Biological Complex Drugs'' (pp. 381-388). Springer International Publishing.Borchard, G., Flühmann, B., & Mühlebach, S. (2012). Nanopa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopharmaceutical
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine. Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms ''biolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Sucrose
Intravenous iron sucrose is a commonly used treatment for iron deficiency anemia. Iron sucrose replaces iron in the blood to foster red blood cell production in patients with chronic kidney disease. Iron sucrose has the trade name Venofer. Chemical structure The chemical formula of iron sucrose is C12H29Fe5Na2O23. The iron sucrose molecule is a polymer with two main molecules; sucrose (chemical formula C12H22O11) and an iron (III) hydroxide (Na2Fe5O8•3(H2O)). These two components are in solution together, but are not bound to one another. Iron sucrose is a type II complex, with two oxygen atoms bonded to each iron atom. When used for medicinal purposes, the iron complex is polymerized and the sucrose molecules combined to form a larger polysaccharide. The number of polymerizations does not have to be the same as the number of sucrose molecules in the polysaccharide. History Iron sucrose's first known use was in Europe in 1949, but it was not used in US medicine until Nov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
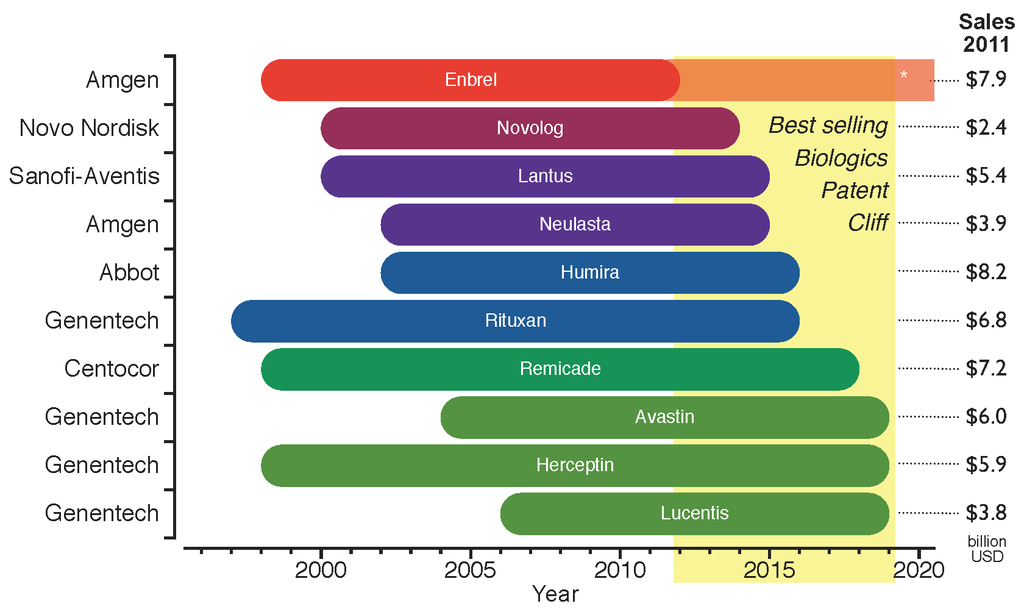
Biosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada hold th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology. A more generalized description of nanotechnology was subsequently established by the National Nanotechnology Initiative, which defined nanotechnology as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). This definition reflects the fact that quantum mechanical effects are important at this quantum-realm scale, and so the definition shifted from a particular technological goal to a research category inclusive of all types of research and technologies that deal with the special properties of matter which occur below the given size threshold. It is therefore commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)