|
Microbial Electrochemical Technologies
Microbial electrochemical technologies (METs) use microorganisms as electrochemical catalyst, merging the microbial metabolism with electrochemical processes for the production of bioelectricity, biofuels, H2 and other valuable chemicals. Microbial fuel cells (MFC) and microbial electrolysis cells (MEC) are prominent examples of METs. While MFC is used to generate electricity from organic matter typically associated with wastewater treatment, MEC use electricity to drive chemical reactions such as the production of H2 or methane. Recently, microbial electrosynthesis cells (MES) have also emerged as a promising MET, where valuable chemicals can be produced in the cathode compartment. Other MET applications include microbial remediation cell, microbial desalination cell, microbial solar cell, microbial chemical cell, etc.,. History The use of microbial cells to produce electricity was perceived by M.C. Potter in 1911 with the finding that "''The disintegration of organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in older texts. The informal synonym ''microbe'' () comes from μικρός, mikrós, "small" and βίος, bíos, "life". is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from ancient times, such as in Jain scriptures from sixth century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells. A common example of a galvanic cell is a standard 1.5 volt cell meant for consumer use. A ''battery'' consists of one or more cells, connected in parallel, series or series-and-parallel pattern. Electrolytic cell An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy. They are often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means ''to break up''. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumaric Acid
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer. Biosynthesis and occurrence It is produced in eukaryotic organisms from succinate in complex 2 of the electron transport chain via the enzyme succinate dehydrogenase. It is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are ''trans'' (''E'') and in maleic acid they are ''cis'' (''Z''). Fumaric acid is found in fumitory (''Fumaria officinalis''), bolete mushrooms (specifically ''Boletus fomentarius var. pseudo-igniarius''), lichen, and Iceland moss. Fumarate is an intermediate in the citric acid cycle us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
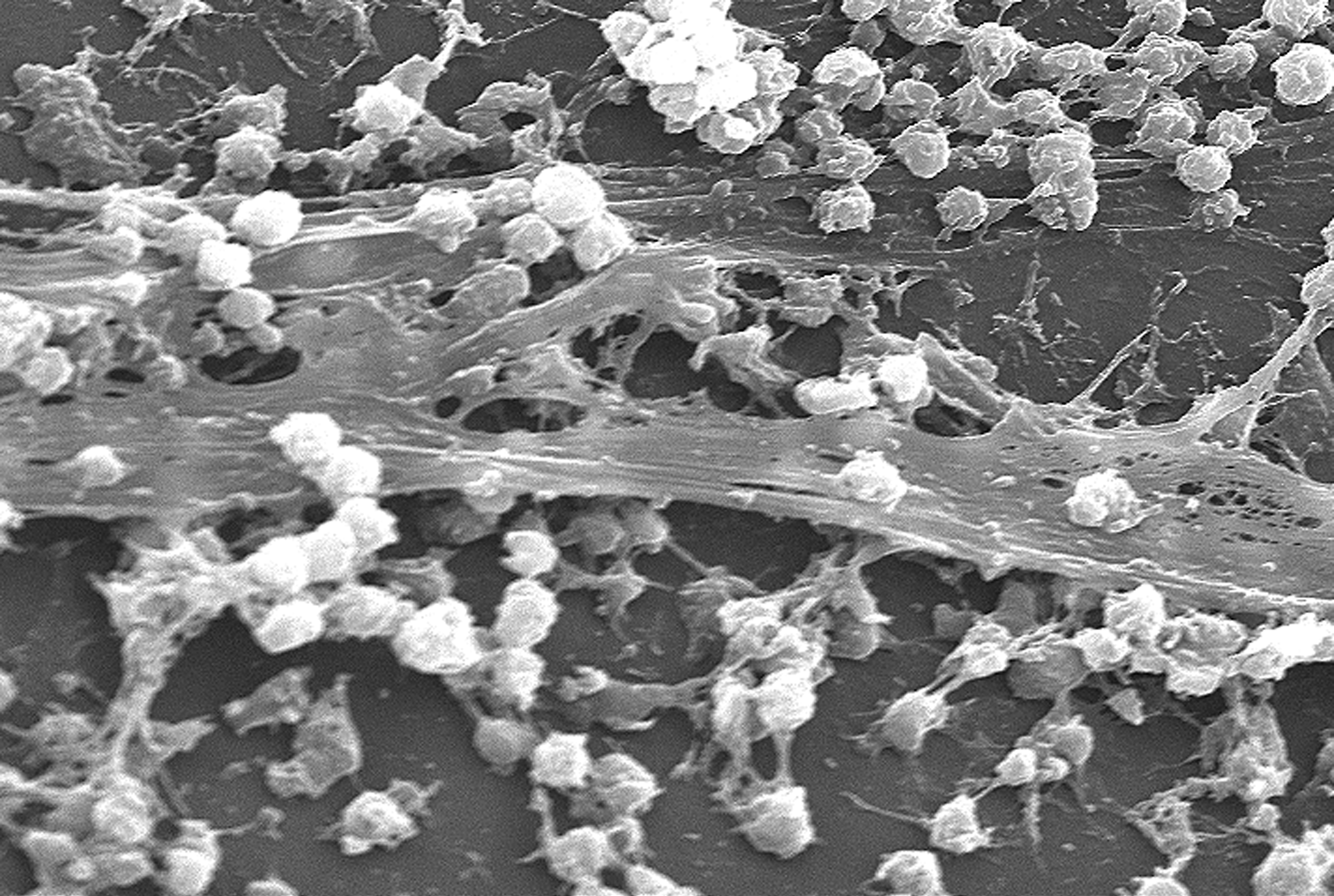
Biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes". Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial, and hospital settings. They may constitute a microbiome or be a portion of it. The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single cells that may float or swim in a liquid medium. Biofilms can form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electricity Generation
Electricity generation is the process of generating electric power from sources of primary energy. For utilities in the electric power industry, it is the stage prior to its delivery ( transmission, distribution, etc.) to end users or its storage (using, for example, the pumped-storage method). Electricity is not freely available in nature, so it must be "produced" (that is, transforming other forms of energy to electricity). Production is carried out in power stations (also called "power plants"). Electricity is most often generated at a power plant by electromechanical generators, primarily driven by heat engines fueled by combustion or nuclear fission but also by other means such as the kinetic energy of flowing water and wind. Other energy sources include solar photovoltaics and geothermal power. There are also exotic and speculative methods to recover energy, such as proposed fusion reactor designs which aim to directly extract energy from intense magnetic fields gene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geobacter Sulfurreducens
''Geobacter sulfurreducens'' is a gram-negative metal and sulphur-reducing proteobacterium. It is rod-shaped, obligately anaerobic, non-fermentative, has flagellum and type four pili, and is closely related to '' Geobacter metallireducens''. ''Geobacter sulfurreducens'' is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, ''G. sulfurreducens'' is one out of twenty different species. The Geobacter genus was discovered by Derek R. Lovley in 1987. ''G. sulfurreducens'' was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch. Characteristics ''Geobacter sulfurreducens'' is a rod-shaped microbe with a gram-negative cell wall. Geobacter is known as a type of bacteria that is able to conduct levels of electricity, and the species ''G. sulfurreducens'' is also known as “electricigens” due to their ability to create an electric current and produce electri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shewanella Oneidensis
''Shewanella oneidensis'' is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name. ''S. oneidensis'' is a facultative bacterium, capable of surviving and proliferating in both aerobic and anaerobic conditions. The special interest in ''S. oneidensis'' MR-1 revolves around its behavior in an anaerobic environment contaminated by heavy metals such as iron, lead and uranium. Experiments suggest it may reduce ionic mercury to elemental mercury and ionic silver to elemental silver. Cellular respiration for these bacteria is not restricted to heavy metals though; the bacteria can also target sulfates, nitrates and chromates when grown anaerobically. Name This species is referred to as ''S. oneidensis'' MR-1, indicating "manganese reducing", a special feature of this organism. It is a common misconception to think that MR-1 refers to "metal-red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shewanella
''Shewanella'' is the sole genus included in the marine bacteria family Shewanellaceae. Some species within it were formerly classed as '' Alteromonas''. ''Shewanella'' consists of facultatively anaerobic Gram-negative rods, most of which are found in extreme aquatic habitats where the temperature is very low and the pressure is very high. ''Shewanella'' bacteria are a normal component of the surface flora of fish and are implicated in fish spoilage. ''Shewanella chilikensis'', a species of the genus ''Shewanella'' commonly found in the marine sponges of Saint Martin's Island of the Bay of Bengal, Bangladesh. ''Shewanella oneidensis'' MR-1 is a widely used laboratory model to study anaerobic respiration of metals and other anaerobic extracellular electron acceptors, and for teaching about microbial electrogenesis and microbial fuel cells. Biochemical characteristics of ''Shewanella'' species Colony, morphological, physiological, and biochemical characteristics of ''Shewanel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing s. It is also common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |