|
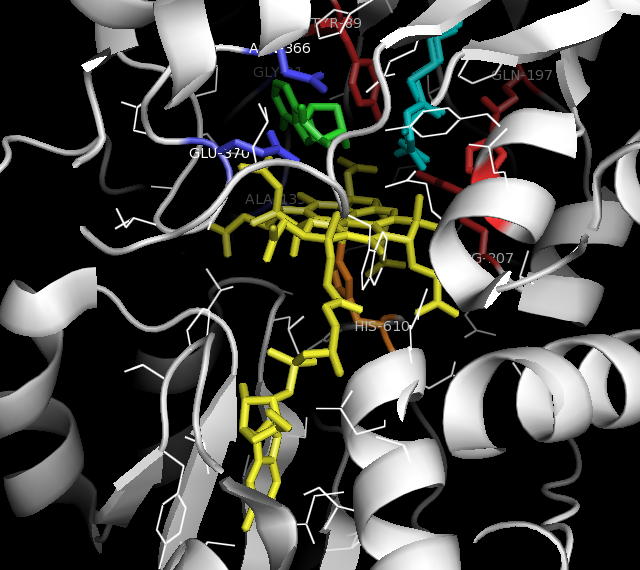
Methylmalonyl-coenzyme A Mutase
Methylmalonyl-CoA mutase (, MCM), mitochondrial, also known as methylmalonyl-CoA isomerase, is a protein that in humans is encoded by the ''MUT'' gene. This vitamin B12-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in ''MUT'' gene may lead to various types of methylmalonic aciduria. MCM was first identified in rat liver and sheep kidney in 1955. In its latent form, it is 750 amino acids in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the N-terminus of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active holoenzyme form. Structure Gene The ''MUT'' gene lies on the chromosome location of 6p12.3 and consists of 13 exons, spanning over 35kb. Protein The mature enzyme is a homodimer with the N-terminal CoA binding domain and the C- terminal cobalam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino acid metabolism. It plays an essential role in the nervous system by supporting myelinogenesis, myelin synthesis and is critical for the maturation of red blood cells in the bone marrow. While animals require B12, plants do not, relying instead on alternative enzymatic pathways. Vitamin B12 is the most chemically complex of all vitamins, and is synthesized exclusively by certain archaea and bacteria. Natural food sources include meat, shellfish, liver, fish, poultry, Egg as food, eggs, and dairy products. It is also added to many breakfast cereals through food fortification and is available in dietary supplement and pharmaceutical forms. Supplements are commonly taken orally but may be administered via intramuscular injection to treat defic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in the human diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from a pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA. Metabolism Biosynthesis In plants and microorganisms, isoleucine is synthesized from pyruvate and alpha-ketobutyrate. This pathway ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonyl-CoA Mutase Deficiency
Methylmalonyl-CoA is the thioester consisting of coenzyme A linked to methylmalonic acid. It is an important intermediate in the biosynthesis of succinyl-CoA, which plays an essential role in the tricarboxylic acid cycle (aka the Citric Acid Cycle, or Krebs Cycle). Biosynthesis and metabolism Methylmalonyl-CoA results from the metabolism of fatty acid with an odd number of carbons, of amino acids valine, isoleucine, methionine, threonine or of cholesterol side-chains, forming Propionyl-CoA. The latter is also formed from propionic acid, which bacteria produce in the intestine. Propionyl-CoA and bicarbonate are converted to Methylmalonyl-CoA by the enzyme propionyl-CoA Carboxylase. It then is converted into succinyl-CoA by methylmalonyl-CoA mutase (MUT). This reaction is a reversible isomerization. In this way, the compound enters the Citric Acid Cycle. The following diagram demonstrates the aforementioned reaction: Propionyl CoA + Bicarbonate → Methylmal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A. Sources It is an important intermediate in the citric acid cycle, where it is synthesized from Alpha-Ketoglutaric acid, α-ketoglutarate by Alpha-ketoglutarate dehydrogenase, α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added. With B12 as an enzymatic cofactor, it is also synthesized from propionyl coenzymeA, propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation. Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12, vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance. Production There are several different pathways through which propionyl-CoA can be produced: * Propionyl-CoA, a three-carbon structure, is considered to be a minor species of propionic acid. Therefore, odd-number chains of fatty acids a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonyl-CoA
Methylmalonyl-CoA is the thioester consisting of coenzyme A linked to methylmalonic acid. It is an important intermediate in the biosynthesis of succinyl-CoA, which plays an essential role in the tricarboxylic acid cycle (aka the Citric Acid Cycle, or Krebs Cycle). Biosynthesis and metabolism Methylmalonyl-CoA results from the metabolism of fatty acid with an odd number of carbons, of amino acids valine, isoleucine, methionine, threonine or of cholesterol side-chains, forming Propionyl-CoA. The latter is also formed from propionic acid, which bacteria produce in the intestine. Propionyl-CoA and bicarbonate are converted to Methylmalonyl-CoA by the enzyme propionyl-CoA Carboxylase. It then is converted into succinyl-CoA by methylmalonyl-CoA mutase (MUT). This reaction is a reversible isomerization. In this way, the compound enters the Citric Acid Cycle. The following diagram demonstrates the aforementioned reaction: Propionyl CoA + Bicarbonate → Methylmalonyl CoA → Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A. Sources It is an important intermediate in the citric acid cycle, where it is synthesized from Alpha-Ketoglutaric acid, α-ketoglutarate by Alpha-ketoglutarate dehydrogenase, α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added. With B12 as an enzymatic cofactor, it is also synthesized from propionyl coenzymeA, propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation. Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12, vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-methylmalonyl-CoA
Methylmalonyl-CoA is the thioester consisting of coenzyme A linked to methylmalonic acid. It is an important intermediate in the biosynthesis of succinyl-CoA, which plays an essential role in the tricarboxylic acid cycle (aka the Citric Acid Cycle, or Krebs Cycle). Biosynthesis and metabolism Methylmalonyl-CoA results from the metabolism of fatty acid with an odd number of carbons, of amino acids valine, isoleucine, methionine, threonine or of cholesterol side-chains, forming Propionyl-CoA. The latter is also formed from propionic acid, which bacteria produce in the intestine. Propionyl-CoA and bicarbonate are converted to Methylmalonyl-CoA by the enzyme propionyl-CoA Carboxylase. It then is converted into succinyl-CoA by methylmalonyl-CoA mutase (MUT). This reaction is a reversible isomerization. In this way, the compound enters the Citric Acid Cycle. The following diagram demonstrates the aforementioned reaction: Propionyl CoA + Bicarbonate → Methylmalonyl CoA → Suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricarboxylic Acid Cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions that release the energy stored in nutrients through acetyl-CoA oxidation. The energy released is available in the form of ATP. The Krebs cycle is used by organisms that generate energy via respiration, either anaerobically or aerobically (organisms that ferment use different pathways). In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, which are used in other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest metabolism components. Even though it is branded as a "cycle", it is not necessary for metabolites to follow a specific route; at least three alternative pathways of the citric acid cycle are recognized. Its name is derived from the citric acid (a tricarboxylic acid, often called citrate, as the ioniz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acids
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymine
Thymine () (symbol T or Thy) is one of the four nucleotide bases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. In RNA, thymine is replaced by the nucleobase uracil. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calf thymus glands, hence its name. Derivation As its alternate name (5-methyluracil) suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thereby stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate), dTDP, or dTTP (for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |