|
Methanol Reformer
A methanol reformer is a device used in chemical engineering, especially in the area of fuel cell technology, which can produce pure hydrogen gas and carbon dioxide by reacting a methanol and water (steam) mixture. :\mathrm \Delta H_^0 = 49.2\ \mathrm Methanol is transformed into hydrogen and carbon dioxide by pressure and heat and interaction with a catalysis, catalyst. Technology A mixture of water and methanol with a molar concentration ratio (water:methanol) of 1.0 - 1.5 is pressurized to approximately 20 bar (unit), bar, vaporized and heated to a temperature of 250 - 360 Celsius, °C. The hydrogen that is created is separated through the use of Pressure swing adsorption or a hydrogen-permeable membrane made of polymer or a palladium alloy. There are two basic methods of conducting this process. * The water-methanol mixture is introduced into a tube-shaped reactor where it makes contact with the catalyst. Hydrogen is then separated from the other r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Engineering
Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials into useful products. Chemical engineering uses principles of chemistry, physics, mathematics, biology, and economics to efficiently use, produce, design, transport and transform energy and materials. The work of chemical engineers can range from the utilization of nanotechnology and nanomaterials in the laboratory to large-scale industrial processes that convert chemicals, raw materials, living cells, microorganisms, and energy into useful forms and products. Chemical engineers are involved in many aspects of plant design and operation, including safety and hazard assessments, process engineering, process design and analysis, modeling and simulation, modeling, control engineering, chemical reaction engineering, nuclear engineering, biologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractory Metals
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definitions of which elements belong to this group differ. The most common definition includes five elements: two of the fifth period (niobium and molybdenum) and three of the sixth period (tantalum, tungsten, and rhenium). They all share some properties, including a melting point above 2000 °C and high hardness at room temperature. They are chemically inert and have a relatively high density. Their high melting points make powder metallurgy the method of choice for fabricating components from these metals. Some of their applications include tools to work metals at high temperatures, wire filaments, casting molds, and chemical reaction vessels in corrosive environments. Partly due to their high melting points, refractory metals are stable against creep deformation to very high tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equipment
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure. Chemical substances can exist in several different physical states or phases (e.g. solids, liquids, gases, or plasma) without changing their chemical composition. Substances transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability are said to be inert. Pure water is an example of a chemical substance, with a constant composition of two hydrogen atoms bonded to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Grove in 1838. The first commercial use of fuel cells came almost a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for satellites and space capsules. Since then, fuel cells hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Production
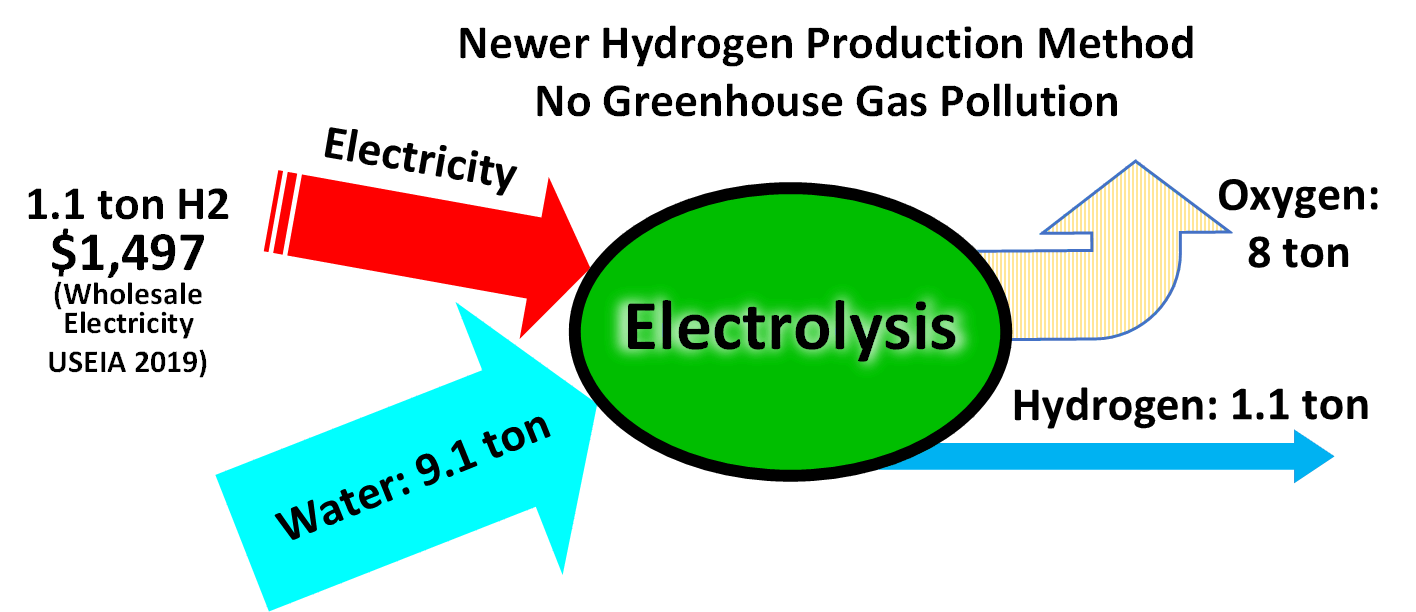
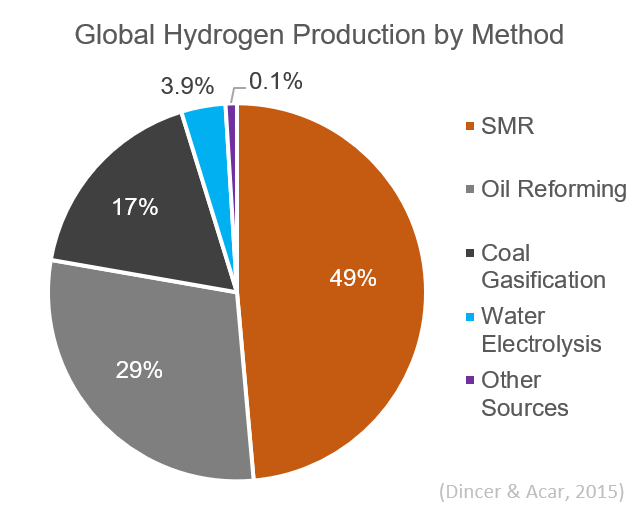
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol Economy
The methanol economy is a suggested future economy in which methanol and dimethyl ether replace fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy, although these concepts are not exclusive. Methanol can be produced from a variety of sources including fossil fuels (natural gas, coal, oil shale, tar sands, etc.) as well as agricultural products and Municipal solid waste, municipal waste, wood and varied biomass. It can also be made from chemical recycling of carbon dioxide. Nobel prize laureate George A. Olah advocated a methanol economy. Uses Fuel Methanol is a fuel for heat engines and fuel cells. Due to its high octane rating it can be used directly as a fuel in flexible-fuel vehicle, flex-fuel cars (including Hybrid electric vehicle, hybrid and plug-in hybrid vehicles) using existing internal combustion engi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PROX
PROX is an acronym for PReferential OXidation, that refers to the preferential oxidation of carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and water-gas shift. An ideal PROX catalyst preferentially oxidizes carbon monoxide (CO) using a heterogeneous catalyst placed upon a ceramic support. Catalysts include metals such as platinum, platinum/iron, platinum/ruthenium, gold nanoparticles as well as novel copper oxide/ceramic conglomerate catalysts. Motivation This reaction is a considerable subject area of research with implications for fuel cell design. Its main utility lies in the removal of carbon monoxide (CO) from the fuel cell's feed gas. CO poisons the catalyst of most low-temperature fuel cells. Carbon monoxide is often produced as a by-product from steam reforming of hydrocarbons, which produces hydrogen and CO. It is possible to consume most of the CO by reacting it with steam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Oxidation
Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between ''thermal partial oxidation'' (TPOX) and ''catalytic partial oxidation'' (CPOX). Principle Partial oxidation is a technically mature process in which natural gas or a heavy hydrocarbon fuel (heating oil) is mixed with a limited amount of oxygen in an exothermic process. * General reaction: + \frac\mathit -> \mathit + \frac\mathitH2 * Idealized reaction for heating oil: + 6O2 -> + 12H2 * Idealized reaction for coal: + 12O2 -> + 6H2 The formulas given for coal and heating oil show only a typical representative of these complex fuels. Water may be added to lower the combustion temperature and reduce soot formation. Yields are below stoichiometric due to some fuel being fully combusted to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of Ammonia production, ammonia or methanol. The reaction is represented by this equilibrium: :CH4 + H2O CO + 3 H2 The reaction is strongly endothermic (Δ''H''SR = 206 kJ/mol). Hydrogen produced by steam reforming is termed grey hydrogen, 'grey' hydrogen when the waste carbon dioxide is released to the atmosphere and blue hydrogen, 'blue' hydrogen when the carbon dioxide is (mostly) captured and stored geologically—see carbon capture and storage. Zero carbon green hydrogen, 'green' hydrogen is produced by Thermochemical cycle, thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Global Warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to Earth's climate. The current rise in global temperatures is driven by human activities, especially fossil fuel burning since the Industrial Revolution. Fossil fuel use, deforestation, and some agricultural and industrial practices release greenhouse gases. These gases absorb some of the heat that the Earth radiates after it warms from sunlight, warming the lower atmosphere. Carbon dioxide, the primary gas driving global warming, has increased in concentration by about 50% since the pre-industrial era to levels not seen for millions of years. Climate change has an increasingly large impact on the environment. Deserts are expanding, while heat waves and wildfires are becoming more common. Amplified warming in the Arctic has c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When formulated as a fuel for engines, gasoline is chemically composed of organic compounds derived from the fractional distillation of petroleum and later chemically enhanced with gasoline additives. It is a high-volume profitable product produced in crude oil refineries. The ability of a particular gasoline blend to resist premature ignition (which causes knocking and reduces efficiency in reciprocating engines) is measured by its octane rating. Tetraethyl lead was once widely used to increase the octane rating but is not used in modern automotive gasoline due to the health hazard. Aviation, off-road motor vehicles, and racing car engines still use leaded gasolines. Other substances are frequently added to gasoline to improve chemical st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daimler-Chrysler
Mercedes-Benz Group AG (formerly Daimler-Benz, DaimlerChrysler, and Daimler) is a German multinational automotive company headquartered in Stuttgart, Baden-Württemberg, Germany. It is one of the world's leading car manufacturers. Daimler-Benz was formed with the merger of Benz & Cie., the world's oldest car company, and Daimler Motoren Gesellschaft in 1926. The company was renamed DaimlerChrysler upon the acquisition of the American automobile manufacturer, Chrysler Corporation in 1998, it was renamed to Daimler upon the divestment of Chrysler in 2007. In 2021, Daimler was the second-largest German automaker and the sixth-largest worldwide by production. In February 2022, Daimler was renamed Mercedes-Benz Group as part of a transaction that spun-off its commercial vehicle segment as an independent company, Daimler Truck. The Mercedes-Benz Group's marques are Mercedes-Benz for cars and vans (including Mercedes-AMG and Mercedes-Maybach). It has shares in other vehicle manufa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |