|
Magnesium Fluoride
Magnesium fluoride is an ionically bonded inorganic compound with the formula . The compound is a colorless to white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite. Production Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride, by the breakdown of it: : Related metathesis reactions are also feasible: : Structure The compound crystallizes as tetragonal birefringent crystals. The structure of the magnesium fluoride is similar to that of rutile, featuring octahedral cations and 3- coordinate anions. In the gas phase, monomeric molecules adopt a linear molecular geometry. Uses Optics Magnesium fluoride is transparent over an extremely wide range of wavelengths. Windows, lenses, and prisms made of this material can be used over the entire range of wavelengths fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sellaite
Sellaite is a magnesium fluoride mineral with the formula MgF2. It crystallizes in the tetragonal crystal system, typically as clear to white vitreous prisms. It may be fibrous and occur as radiating aggregates. It has a Mohs hardness of 5 to 6 and a specific gravity of 2.97 to 3.15. Refractive index values are ''nω'' = 1.378 and ''nε'' = 1.390. Discovery and occurrence Sellaite was first described in 1868 and named for Italian mining engineer and mineralogist Quintino Sella (1827–1884). Its type locality is the in France, where it occurred inside bitumen-bearing dolomite-anhydrite clasts within a moraine deposit. It has been reported in an evaporite deposit at Bleicherode; within volcanic ejecta and fumaroles at Vesuvius; in a metamorphic magnesite deposit at ; and in sodic alkali granite Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Fluoride
Aluminium fluoride is an inorganic compound with the formula . It forms hydrates . Anhydrous and its hydrates are all colorless solids. Anhydrous is used in the production of aluminium. Several occur as minerals. Occurrence and production Aside from anhydrous , several hydrates are known. With the formula , these compounds include monohydrate (''x'' = 1), two polymorphs of the trihydrate (''x'' = 3), a hexahydrate (''x'' = 6), and a nonahydrate (''x'' = 9). The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride at 700 °C: Hexafluorosilicic acid may also be used make aluminium fluoride. : Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, can also be prepared by treating aluminium hydroxide or aluminium with hydrogen fluoride. Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite. The anhydrous form appears as the relatively ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
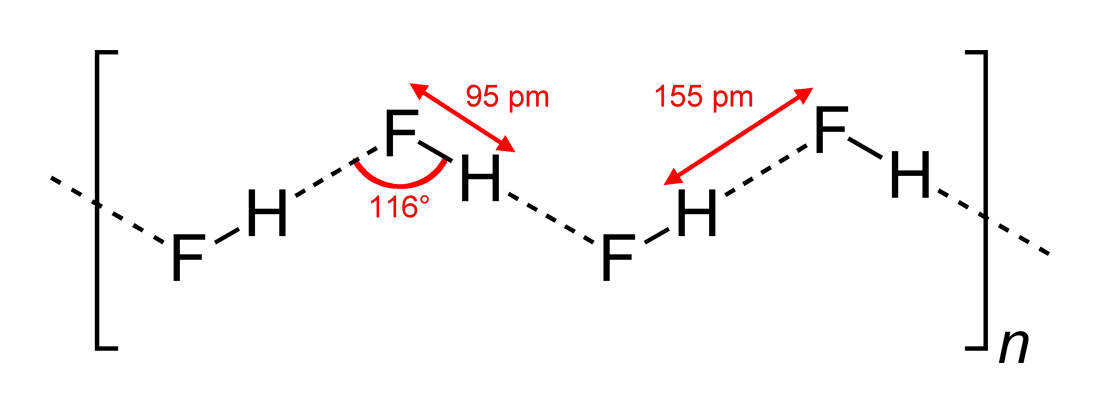
Ammonium Bifluoride
Ammonium bifluoride is an inorganic compound with the formula or . It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-industrial etching, etchant and an intermediate in a once-contemplated route to hydrofluoric acid. Structure Ammonium bifluoride, as its name indicates, contains an ammonium cation (), and a bifluoride (or hydrogen difluoride) anion (). The triatomic bifluoride anion features a strong three-center four-electron bond (specifically, a symmetrical hydrogen bond) with a bond energy greater than 155 kJ/mol, and an H-F length of 114 pm. In solid form (), ammonium biflouride is similar to other fluoride salts. Its crystal system is considered orthorhombic, with each cation coordinated with four anions in a tetrahedron (and vice versa). Hydrogen atoms in the ammonium ion form hydrogen bonds with the fluorine atoms, and in the resulting structure, N-H-F are roughly colinear. As a result of these hydrogen bonds, this crystal structure varie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia nigra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important Reagent, chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sellaite
Sellaite is a magnesium fluoride mineral with the formula MgF2. It crystallizes in the tetragonal crystal system, typically as clear to white vitreous prisms. It may be fibrous and occur as radiating aggregates. It has a Mohs hardness of 5 to 6 and a specific gravity of 2.97 to 3.15. Refractive index values are ''nω'' = 1.378 and ''nε'' = 1.390. Discovery and occurrence Sellaite was first described in 1868 and named for Italian mining engineer and mineralogist Quintino Sella (1827–1884). Its type locality is the in France, where it occurred inside bitumen-bearing dolomite-anhydrite clasts within a moraine deposit. It has been reported in an evaporite deposit at Bleicherode; within volcanic ejecta and fumaroles at Vesuvius; in a metamorphic magnesite deposit at ; and in sodic alkali granite Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Telescope
A space telescope (also known as space observatory) is a telescope in outer space used to observe astronomical objects. Suggested by Lyman Spitzer in 1946, the first operational telescopes were the American Orbiting Astronomical Observatory, OAO-2 launched in 1968, and the Soviet Orion (space telescope), Orion 1 ultraviolet telescope aboard space station Salyut 1 in 1971. Space telescopes avoid several problems caused by the atmosphere, including the absorption or scattering of certain wavelengths of light, obstruction by clouds, and distortions due to atmospheric refraction such as twinkling. Space telescopes can also observe dim objects during the daytime, and they avoid light pollution which Observatory#Ground-based observatories, ground-based observatories encounter. They are divided into two types: Satellites which map the entire sky (astronomical survey), and satellites which focus on selected astronomical objects or parts of the sky and beyond. Space telescopes are distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optics
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of optical instruments, instruments that use or Photodetector, detect it. Optics usually describes the behaviour of visible light, visible, ultraviolet, and infrared light. Light is a type of electromagnetic radiation, and other forms of electromagnetic radiation such as X-rays, microwaves, and radio waves exhibit similar properties. Most optical phenomena can be accounted for by using the Classical electromagnetism, classical electromagnetic description of light, however complete electromagnetic descriptions of light are often difficult to apply in practice. Practical optics is usually done using simplified models. The most common of these, geometric optics, treats light as a collection of Ray (optics), rays that travel in straight lines and bend when they pass through or reflect from surfaces. Physical optics is a more comprehensive mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats. In other words, it is the distance between consecutive corresponding points of the same ''phase (waves), phase'' on the wave, such as two adjacent crests, troughs, or zero crossings. Wavelength is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The multiplicative inverse, inverse of the wavelength is called the ''spatial frequency''. Wavelength is commonly designated by the Greek letter lambda (''λ''). For a modulated wave, ''wavelength'' may refer to the carrier wavelength of the signal. The term ''wavelength'' may also apply to the repeating envelope (mathematics), envelope of modulated waves or waves formed by Interference (wave propagation), interference of several sinusoids. Assuming a sinusoidal wave moving at a fixed phase velocity, wave speed, wavelength is inversely proportion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |