|
Liquid Scintillation Counting
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator (e.g. zinc sulfide), and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection. Technique Samples are dissolved or suspended in a "cocktail" containing a solvent (historically aromatic organics such as xylene or toluene, but more recently less hazardous solvents are used), typically some form of a surfactant, and "fluors" or scintillators which produce the light measured by the detector. Scintillators can be divided into primary and secondary phosphors, differing in their luminescence properties. Beta particles emitted from the isotopic sample transfer energy to the solvent molecules: the π cloud of the aromatic ring absorbs the energy of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
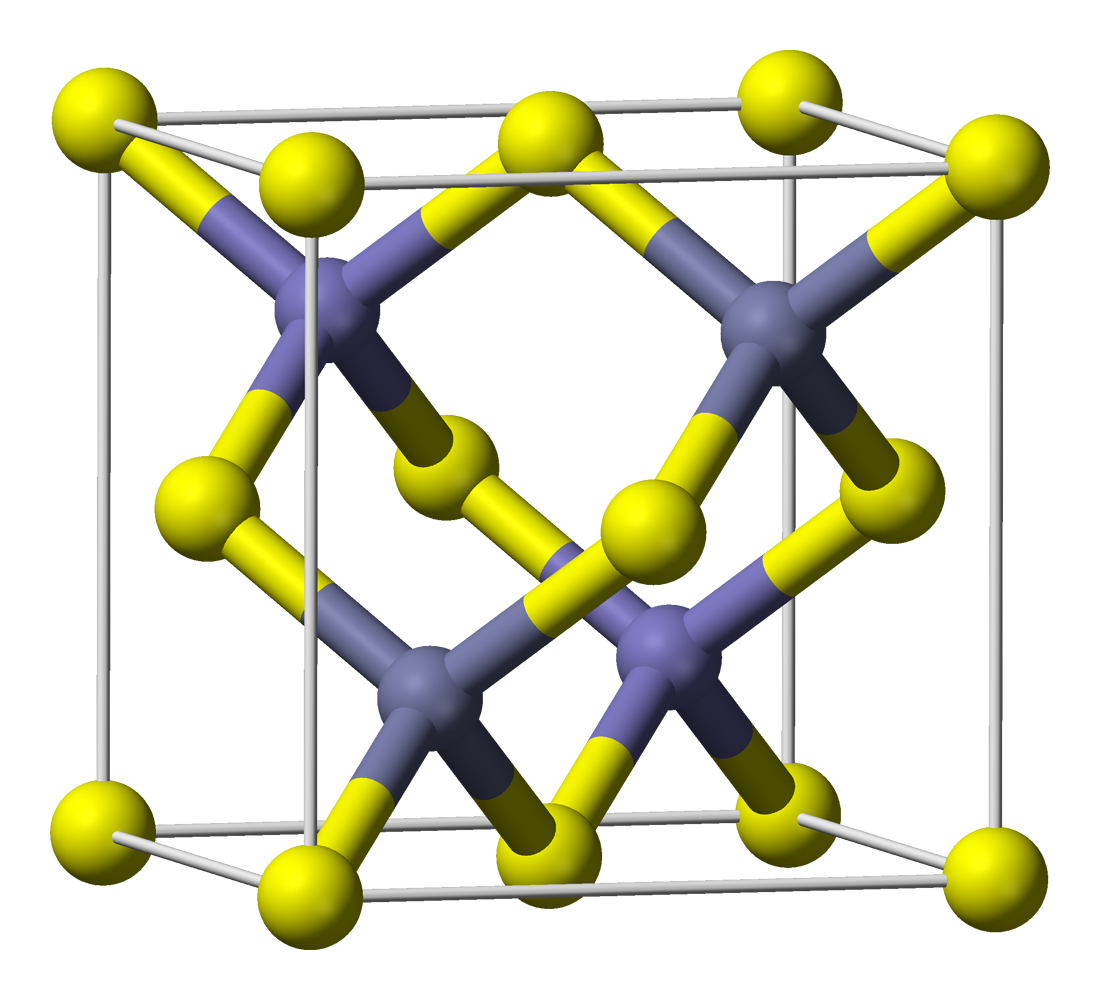
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Appli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translucent
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) allows light to pass through, but does not necessarily (again, on the macroscopic scale) follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Counting Efficiency
In the measurement of ionising radiation the counting efficiency is the ratio between the number of particles or photons counted with a radiation counter and the number of particles or photons of the same type and energy emitted by the radiation source. Factors Several factors affect the counting efficiency: * The distance from the source of radiation * The absorption or scattering of particles by the medium (such as air) between the source and the surface of the detector * The detector efficiency in counting all radiation photons and particles that reach the surface of the detector The accompanying diagram shows this graphically. Scintillation counters Radiation protection instruments Large area scintillation counters used for surface radioactive contamination measurements use plate or planar radioactive sources as calibration standards. The Surface Emission Rate (SER), not the source activity, is used as a measure of the rate of particles emitted from the source of radiation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerator Mass Spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the mass spectrometric methods is its power to separate a rare isotope from an abundant neighboring mass ("abundance sensitivity", e.g. 14C from 12C). The method suppresses molecular isobars completely and in many cases can separate atomic isobars (e.g. 14N from 14C) also. This makes possible the detection of naturally occurring, long-lived radio-isotopes such as 10Be, 36Cl, 26Al and 14C. Their typical isotopic abundance ranges from 10−12 to 10−18. AMS can outperform the competing technique of decay counting for all isotopes where the half-life is long enough. Other advantages of AMS include its short measuring time as well as its ability to detect atoms in extremely small samples. The method Generally, negative ions are created (atoms are ionized) in an ion source. In for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium-90
Yttrium-90 () is an isotope of yttrium. Yttrium-90 has found a wide range of uses in radiation therapy to treat some forms of cancer. Decay undergoes β− decay to zirconium-90 with a half-life of 64.1 hours and a decay energy of 2.28 MeV with an average beta energy of 0.9336 MeV. It also produces 0.01% 1.7 MeV photons during its decay process to the 0+ state of 90Zr, followed by pair production. The interaction between emitted electrons and matter can lead to the emission of Bremsstrahlung radiation. Production Yttrium-90 is produced by the nuclear decay of strontium-90 which has a half-life of nearly 29 years and is a fission product of uranium used in nuclear reactors. As the strontium-90 decays, chemical high-purity separation is used to isolate the yttrium-90 before precipitation. Medical application 90Y plays a significant role in the treatment of hepatocellular carcinoma (HCC), leukemia, and lymphoma, although it has the potential to treat a range of tumo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus-32
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons, one more neutron than the most common isotope of phosphorus, phosphorus-31. Phosphorus-32 only exists in small quantities on Earth as it has a short half-life of 14 days and so decays rapidly. Phosphorus is found in many organic molecules and so phosphorus-32 has many applications in medicine, biochemistry, and molecular biology where it can be used to trace phosphorylated molecules (for example, in elucidating metabolic pathways) and radioactively label DNA. Decay Phosphorus-32 has a short half-life of 14.268 days and decays into sulfur-32 by beta decay as shown in this nuclear equation: : 1.709 MeV of energy is released during the decay. The kinetic energy of the electron varies with an average of approximately 0.5 MeV and the remainder of the energy is carried by the nearly undetectable electron antineutrino. In comparison to other beta radiation- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval alchemists, which commonly involved the heating of chloride salts like ammonium chloride ( sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However, the nature of free chlorine gas as a separate substance was only recognised aroun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Phosphorus
Although phosphorus (15P) has 23 isotopes from 25P to 47P, only 31P is stable; as such, phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable is 25P with a half-life shorter than 30 nanoseconds. List of isotopes , - , rowspan=3, 24P?The existence of this isotope has not been experimentally confirmed; given data is inferred or estimated from periodic trends. , rowspan=3 style="text-align:right" , 15 , rowspan=3 style="text-align:right" , 9 , rowspan=3, 24.03652(54)# , rowspan=3, , p? , 23Si , rowspan=3, 1+# , rowspan=3, , rowspan=3, , - , β+? , 24Si , - , β+, p? , 23Al , - , 25P? , style="text-align:right" , 15 , style="text-align:right" , 10 , 25.02119(43)# , <30 ns , p? , 24Si , (1/2+)# , , , - , rowspan=2, 26P [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Line Noise
In electronics, noise is an unwanted disturbance in an electrical signal. Noise generated by electronic devices varies greatly as it is produced by several different effects. In particular, noise is inherent in physics, and central to thermodynamics. Any conductor with electrical resistance will generate thermal noise inherently. The final elimination of thermal noise in electronics can only be achieved cryogenically, and even then quantum noise would remain inherent. Electronic noise is a common component of noise in signal processing. In communication systems, noise is an error or undesired random disturbance of a useful information signal in a communication channel. The noise is a summation of unwanted or disturbing energy from natural and sometimes man-made sources. Noise is, however, typically distinguished from interference, for example in the signal-to-noise ratio (SNR), signal-to-interference ratio (SIR) and signal-to-noise plus interference ratio (SNIR) mea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coincidence Circuit
In physics and electrical engineering, a coincidence circuit or coincidence gate is an electronic device with one output and two (or more) inputs. The output activates only when the circuit receives signals within a time window accepted as ''at the same time'' and in parallel at both inputs. Coincidence circuits are widely used in particle detectors and in other areas of science and technology. Walther Bothe shared the Nobel Prize for Physics in 1954 "...for his discovery of the method of coincidence and the discoveries subsequently made by it." Bruno Rossi invented the electronic coincidence circuit for implementing the coincidence method. History Bothe, 1924 In his Nobel Prize lecture,{{cite web , url=http://nobelprize.org/nobel_prizes/physics/laureates/1954/bothe-lecture.html , title=Nobel Lecture , author=Bothe, Walther , year=1954 , publisher= Nobel Foundation Bothe described how he had implemented the coincidence method in an experiment on Compton scattering in 1924. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |