|
List Of Compounds With Carbon Number 12
This is a partial list of molecules that contain 12 carbon atoms. C12H0 – C12H8 C12H9 – C12H11 C12H12 – C12H14 C12H15 – C12H17 C12H18 – C12H19 C12H20 – C12H21 C12H22 C12H23 – C12H27 C12H28 – C12Hmany {, class="wikitable" , - , C12H28BF4N , , tetrapropylammonium tetrafluoroborate , , 15553-52-3 , - , C12H28Ge , , tetrapropyl germane , , 994-65-0 , - , C12H28N2 , , tetrapropylhydrazine , , 60678-69-5 , - , C12H28OSi , , hexyloxytriethylsilane , , 18107-40-9 , - , C12H28O4Si , , tetraisopropoxysilane , , 1992-48-9 , - , C12H28O4Si , , tetrapropyl silicate , , 682-01-9 , - , C12H30OSi2 , , hexaethyldisiloxane , , 994-49-0 , - , C12H36ClCr3O22 , , triaquo hexacetate chromate chloride hexahydrate , , 12366-60-8 , - , C12H36Li4Si4 , , trimethylsilyllithium tetramer , , 18000-27-6 , - , C12H36O4Si5 , , dodecamethylpentasiloxane , , 141-63-9 , - , C12H36O6Si6 , , dodecamethylcyclohexasiloxane , , 540-97-6 , - , C12N6 , , h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentabromodiphenyl Ether
Pentabromodiphenyl ether (also known as pentabromodiphenyl oxide) is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP). Composition, uses, and production Commercial pentaBDE is a technical mixture of different PBDE congeners, with BDE-47 (2,2',4,4'- tetrabromodiphenyl ether) and BDE-99 (2,2',4,4',5-pentabromodiphenyl ether) as the most abundant.Persistent Organic Pollutants Review Committee of the Stockholm ConventionCommercial Pentabromodiphenyl Ether: Risk Management Evaluation.United Nations Environment Programme, August 2007. The term pentaBDE alone refers to isomers of pentabromodiphenyl ether (PBDE congener numbers 82-127).Agency for Toxic Substances and Disease RegistryToxicological Profile for Polybrominated Biphenyls and Polybro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
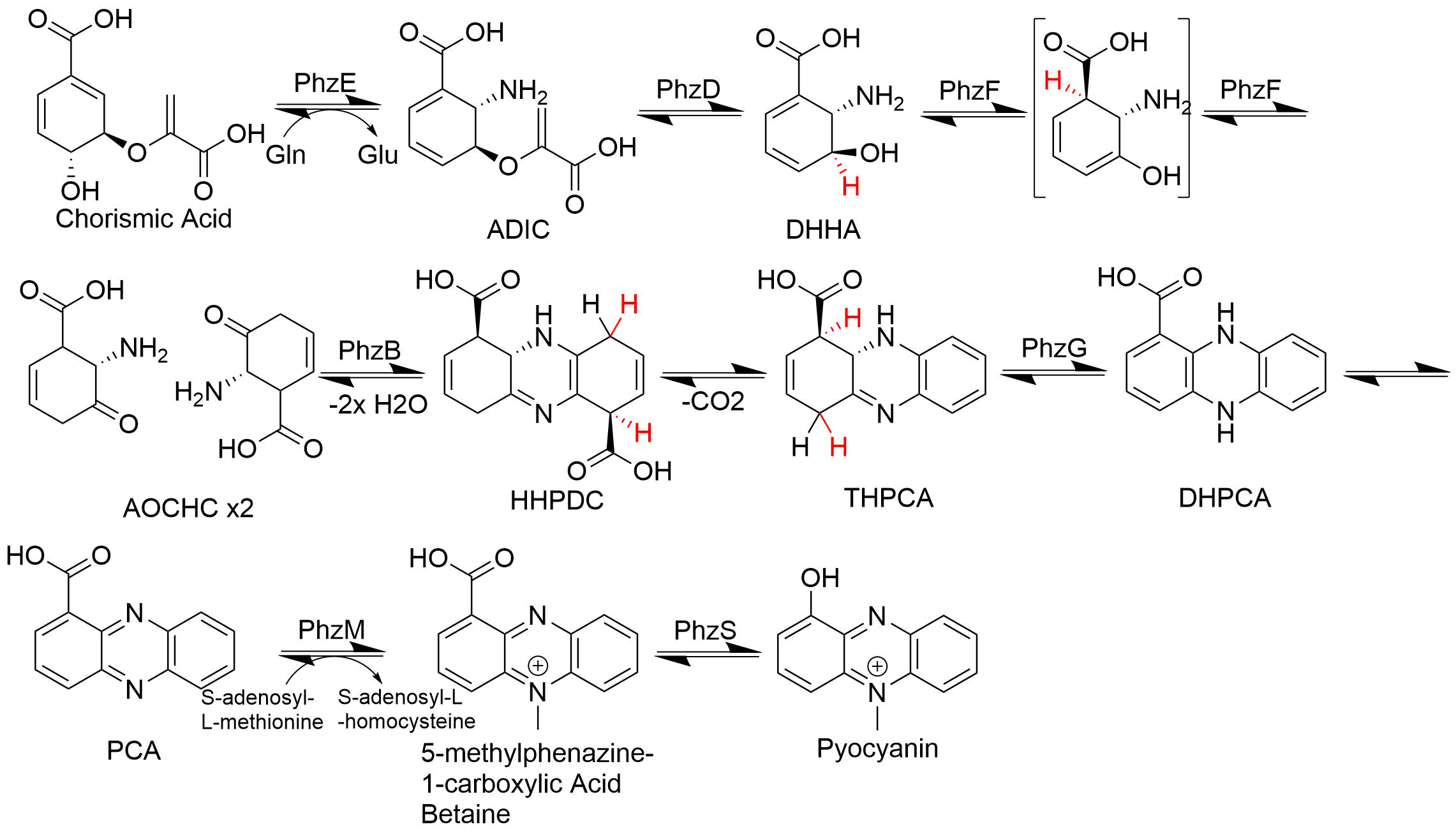
Phenazine
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution. Synthesis Classically phenazine are prepared by the reaction of nitrobenzene and aniline in the Wohl-Aue reaction. Other methods include: * pyrolysis of the barium salt of azobenzoate * oxidation of aniline with lead oxide * oxidation of dihydrophenazine, which is prepared by heating pyrocatechin with o-phenylenediamine. * oxidation of ortho-aminodiphenylamine with lead peroxide. Derivatives * The more complex phenazines, such as the naphthophenazines, naphthazines, and naphthotolazines, may be prepared by condensing ortho-diamines with ortho-quinones or by the oxidation of an ortho-diamine in the pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endrin
Endrin is an organochloride with the chemical formula C12H8Cl6O that was first produced in 1950 by Shell and Velsicol Chemical Corporation. It was primarily used as an insecticide, as well as a rodenticide and piscicide. It is a colourless, odorless solid, although commercial samples are often off-white. Endrin was manufactured as an emulsifiable solution known commercially as Endrex. The compound became infamous as a persistent organic pollutant and for this reason it is banned in many countries. In the environment endrin exists as either endrin aldehyde or endrin ketone and can be found mainly in bottom sediments of bodies of water. Exposure to endrin can occur by inhalation, ingestion of substances containing the compound, or skin contact. Upon entering the body, it can be stored in body fats and can act as a neurotoxin on the central nervous system, which can cause convulsions, seizures, or even death. Although endrin is not currently classified as a mutagen, nor as a hum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldrin
Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after The Second World War. Other noteworthy examples of COCs include DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides (a term for pesticides derived from Hexachlorocyclopentadiene) became notorious as persistent organic pollutants.Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Weinheim, 2002. Structure & Reactivity The structure formula of aldrin is C12H8Cl6. The molecule has a molecular weight of 364.896 g/mol. The melting point of aldri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenylene
Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon. Bonding Biphenylene is a polycyclic hydrocarbon, composed of two benzene rings joined by two bridging bonds (as opposed to a normal ring fusion), thus forming a 6-4-6 arene system. The resulting planar structure was one of the first π-electronic hydrocarbon systems discovered to show evidence of antiaromaticity. The spectral and chemical properties show the influence of the central nring, leading to considerable interest in the system in terms of its degree of lessened aromaticity. Questions of bond alternation and ring currents have been investigated repeatedly. Both X-ray diffraction and electron diffraction studies show a considerable alternation of bond lengths, with the bridging bonds between the benzenoid rings having the unusually great length of 1.524 Å. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acenaphthylene
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence. Occurrence Acenaphthylene occurs as about 2% of coal tar. It is produced industrially by gas phase dehydrogenation of acenaphthene. Reactions Hydrogenation gives the more saturated compound acenaphthene Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound. Production and reactions Acena .... Chemical reduction affords the radical anion sodium or potassium acenaphthalenide, which is used as a strong reductant (E = -2.26 V vs FC).N. G. Connelly and W. E. Geiger, "Chemical Redox Agents for Organometallic Chemistry", Chem. Rev. 1996, 96, 877-910 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenehexacarboxylic Acid
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky needles and is soluble in water and alcohol. Preparation Mellitic acid may be prepared by warming mellite with ammonium carbonate, boiling off the excess of the ammonium salt, and adding ammonia to the solution. The precipitated alumina is filtered off, the filtrate evaporated, and the ammonium salt of the acid purified by recrystallization. The ammonium salt is then converted into the lead salt by precipitation with lead acetate, and the lead salt is then decomposed by hydrogen sulfide. The acid may also be prepared by the oxidation of pure carbon, graphite or hexamethylbenzene, by alkaline potassium permanganate in the cold, or by hot concentrated nitric acid. Reactions It is a very stable compound; chlorine, concentrated nitric ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobenzene Complex
Hexafluorobenzene, HFB, , or perfluorobenzene is an organic, aromatic compound. In this derivative of benzene all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although it is recommended as a solvent in a number of photochemical reactions. In the laboratory hexafluorobenzene is used as standard in fluorine-19 NMR spectroscopy, solvent and standard in carbon-13 NMR, solvent in proton NMR, solvent when studying some parts in the infrared and solvent in ultraviolet–visible spectroscopy, as hexafluorobenzene itself hardly shows any absorbance in the UV region. Geometry of the aromatic ring Hexafluorobenzene stands somewhat aside in the perhalogenbenzenes. When counting for bond angles and distances it is possible to calculate the distance between two ortho fluorine atoms. Also the non bonding radius of the halogens is known. The following table presents the results: The conclusion of the table is hexafluorobenzene is the o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bithionol
Bithionol is an antibacterial, anthelmintic, and algaecide. It is used to treat ''Anoplocephala perfoliata'' (tapeworms) in horses and ''Fasciola hepatica'' (liver flukes). __TOC__ Mechanism of action Bithionol has been shown to be a potent inhibitor of soluble adenylyl cyclase, an intracellular enzyme important in the catalysis of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Soluble adenylyl cyclase is uniquely activated by bicarbonate. The cAMP formed by this enzyme is associated with capacitation of sperm, eye pressure regulation, acid-base regulation, and astrocyte/neuron communication. It is related to the organochlorine hexachlorophene, which has been shown to be an isomer-specific inhibitor of soluble adenylyl cyclase. Bithionol has two aromatic rings with a sulfur atom bonded between them and multiple chlorine ions and hydroxyl groups attached to the phenyl groups. These functional groups are capable of hydrophobic, ionic, and polar inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |