|
List Of UN Numbers 1001 To 1100
UN numbers from UN1001 to UN1100 as assigned by the United Nations Committee of Experts on the Transport of Dangerous Goods are as follows: __NOTOC__ UN 1001 to UN 1100 n.o.s. = ''not otherwise specified'' meaning a collective entry to which substances, mixtures, solutions or articles may be assigned if a) they are not mentioned by name in ''3.2 Dangerous Goods List'' AND b) they exhibit chemical, physical and/or dangerous properties corresponding to the Class, classification code, packing group and the name and description of the n.o.s. entry See also * Lists of UN numbers References External linksADR Dangerous Goods cited on 2 June 2015.UN Dangerous Goods List from 2015 cited on 2 June 2015.UN Dangerous Goods List from 2013 cited on 2 June 2015. {{UN number list navbox Lists of UN numbers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is Saturated and unsaturated compounds, unsaturated because its two carbon atoms are Chemical bond, bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. The triple bond in acetylene results in a high energy content that is released when acetylene is burned. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Chloro-1,2,2,2-tetrafluoroethane
1-Chloro-1,2,2,2-tetrafluoroethane, C2 H Cl F4, is a hydrochlorofluorocarbon used as a component in refrigerants offered as replacements for chlorofluorocarbons. HCFC-124 is also used in gaseous fire suppression Gaseous fire suppression, also called clean agent fire suppression, is the use of inert gases and chemical agents to extinguish a fire. These agents are governed by the National Fire Protection Association (NFPA) Standard for Clean Agent Fire Ex ... systems as a replacement for bromochlorocarbons.DuPont HCFC-124 Properties, Uses, Storage, and Handling. DuPont Fluorochemicals, Wilmington, DE. 2005 References Refrigerants Hydrochlorofluorocarbons Ozone-depleting chemical substances Chlorofluoroalkanes {{Organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods in chemistry, isolated on an industrial scale from natural gas and as a petrochemical by-product of oil refinery, petroleum refining. Its chief use is as feedstock for ethylene production. The ethyl group is formally, although rarely practically, derived from ethane. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. The process is now called Kolbe electrolysis: : acetate, CH3COO− → CH3• + carbon dioxide, CO2 + electron, e− : CH3• + •CH3 → C2H6 During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3, (sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. Dimethyl ether was first synthesised by Jean-Baptiste Dumas and Eugene Péligot in 1835 by distillation of methanol and sulfuric acid. Production Approximately 50,000 tons were produced in 1985 in Western Europe by dehydration of methanol:Manfred Müller, Ute Hübsch, "Dimethyl Ether" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. : The required methanol is obtained from synthesis gas ( syngas). Other possible improvements call for a dual catalyst system that permits both methanol synthesis and dehydration in the same process unit, with no methanol isolation and purificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one hydrogen. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: : Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of a few mg/kg. Uses Dimethylamine is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-Difluoroethane
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula CHF. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential than other hydrofluorocarbons and a shorter atmospheric lifetime (1.4 years). Production 1,1-Difluoroethane is a synthetic substance that is produced by the mercury-catalyzed addition of hydrogen fluoride to acetylene: :HCCH + 2 HF → CHCHF The intermediate in this process is vinyl fluoride (C2H3F), the monomeric precursor to polyvinyl fluoride. Uses With a relatively low global warming potential (GWP) index of 124 and favorable thermophysical properties, 1,1-difluoroethane has been proposed as an environmentally friendly alternative to R134a. Despite its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorofluoromethane
Dichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon with the formula CHCl2F. It is a colorless and odorless gas. It is produced by fluorination of chloroform using a catalyst such as antimony trifluoride: :CHCl3 + HF → CHCl2F + HCl Uses Dichlorofluoromethane was used as a propellant and refrigerant. Due to its role in ozone depletion, dichlorofluoromethane has been largely phased out. It has ozone depletion potential 0.04. Production and consumption has been since 2004 reduced to 15% of level from 1989 and it is to be phased out in 2015 according to Montreal Protocol. Pyrolysis of a mixture of dichlorofluoromethane and chlorofluoromethane gives hexafluorobenzene: :3 CHCl2F + 3 CH2ClF → C6F6 + 9 HCl Additional physical data Its critical point is at 178.5 °C (451.7K) and 5.17MPa (51.7bar). At temperatures from 5K to 105K, it has one phase in the space group Pbca. Safety Its toxicity is comparable to that of chloroform Chloroform, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorodifluoromethane
Dichlorodifluoromethane (R-12) is a colorless gas popularly known by the genericized brand name Freon (as Freon-12). It is a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. In compliance with the Montreal Protocol, its manufacture was banned in developed countries (non-article 5 countries) in 1996, and in developing countries (Article 5 countries) in 2010 out of concerns about its damaging effect on the ozone layer. Its only allowed usage is as a fire retardant in submarines and aircraft. It is soluble in many organic solvents. R-12 cylinders are colored white. Preparation It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: :CCl4 + 2HF → CCl2F2 + 2HCl This reaction can also produce trichlorofluoromethane (CCl3F), chlorotrifluoromethane (CClF3) and tetrafluoromethane (CF4). History Charles F. Kettering, vice president of General Motors Researc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
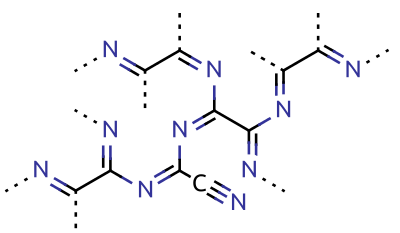
Cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear molecular geometry, linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as chlorine, Cl, but far less oxidizing. The two cyanide, cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |