|
Leda Clay
Quick clay, also known as Leda clay and Champlain Sea clay in Canada, is any of several distinctively sensitive glaciomarine clays found in Canada, Norway, Russia, Sweden, Finland, the United States, and other locations around the world. The clay is so unstable that when a mass of quick clay is subjected to sufficient stress, the material behavior may drastically change from that of a particulate material to that of a watery fluid. Landslides occur because of the sudden soil liquefaction caused by external solicitations such as vibrations induced by an earthquake, or massive rainfalls. Quick clay main deposits Quick clay is found only in countries close to the north pole, such as Russia; Canada; Norway; Sweden; and Finland; and in Alaska (United States); since they were glaciated during the Pleistocene epoch. In Canada, the clay is associated primarily with the Pleistocene-era Champlain Sea, in the modern Ottawa Valley, the St. Lawrence Valley, and the Saguenay River regions. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canada
Canada is a country in North America. Its Provinces and territories of Canada, ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, making it the world's List of countries and dependencies by area, second-largest country by total area, with the List of countries by length of coastline, world's longest coastline. Its Canada–United States border, border with the United States is the world's longest international land border. The country is characterized by a wide range of both Temperature in Canada, meteorologic and Geography of Canada, geological regions. With Population of Canada, a population of over 41million people, it has widely varying population densities, with the majority residing in List of the largest population centres in Canada, urban areas and large areas of the country being sparsely populated. Canada's capital is Ottawa and List of census metropolitan areas and agglomerations in Canada, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L solution) or molal (mol/kg solvent) and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. Quantifying ionic strength The molar ionic strength, ''I'', of a solution is a function of the concentration of ''all'' ions present in that solution. :I = \begin\frac\end\sum_^ c_i z_i^ where one half is because we are including both cations and anions, ''c''i is the molar concentration of ion i (M, mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
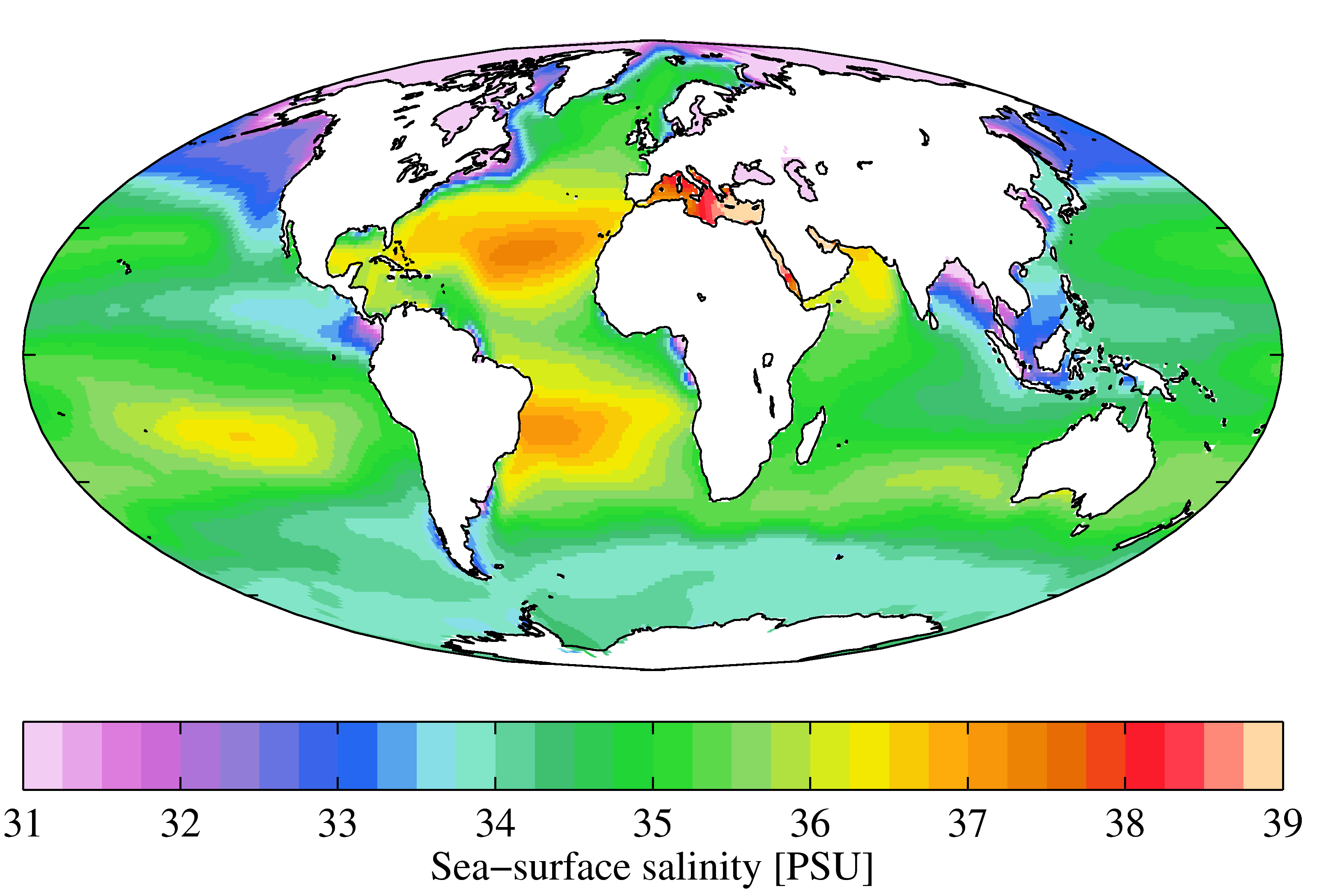
Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to per mille, ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a state function, thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Layer (surface Science)
Double layer may refer to: * Double layer (biospecific), the surface where two different phases of matter are in contact * Double layer (plasma physics), a structure in a plasma and consists of two parallel layers with opposite electrical charge * Double layer (surface science), a structure that appears on the surface of an object when it is placed into a liquid * Double layer forces, which occur between charged objects across liquids * Double layer potential, a solution of Laplace's equation * Double layer suturing, two layers of sutures, first in a deep level of a tissue and then at a more superficial level * DVD+R DL or Double layer, a DVD format {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon chemistry and silicate mineralogy. If a silanol contains one or more organic residues, it is an organosilanol. Preparation From alkoxysilanes The first isolated example of a silanol was , reported in 1871 by Albert Ladenburg. He prepared the “silicol” by hydrolysis of (Et = ). From silyl halides and related compounds Silanols are generally synthesized by hydrolysis of halosilanes, alkoxysilanes, or aminosilanes. Chlorosilanes are the most common reactants: :R3Si–Cl + H2O → R3Si–OH + HCl The hydrolysis of fluorosilanes requires more forcing reagents, i.e. alkali. The alkoxysilanes ( silyl ethers) of the type are slow to hydrolyze. Compared to the silyl ethers, silyl acetates are faster to hydrolyze, with th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protolysis
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation-exchange Capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with other positively charged particles in the surrounding soil water. This is one of the ways that solid materials in soil alter the chemistry of the soil. CEC affects many aspects of soil chemistry, and is used as a measure of soil fertility, as it indicates the capacity of the soil to retain several nutrients (e.g. K+, NH4+, Ca2+) in plant-available form. It also indicates the capacity to retain pollutant cations (e.g. Pb2+). Definition and principles Cation-exchange capacity is defined as the amount of positive charge that can be exchanged per mass of soil, usually measured in cmolc/kg. Some texts use the older, equivalent units me/100g or meq/100g. CEC is measured in moles of electric charge, so a cation-exchange capacity of 10 cmolc/kg co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the Abundance of elements in Earth's crust, sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been Leaching (chemistry), leached by the action of water from the Earth, Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in Soap, soap manufac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Neutrality
Global net-zero emissions is reached when greenhouse gas emissions and removals due to human activities are in balance. It is often called simply net zero. ''Emissions'' can refer to all greenhouse gases or only carbon dioxide (). Reaching net zero is necessary to stop further global warming. It requires deep cuts in emissions, for example by shifting from fossil fuels to sustainable energy, improving energy efficiency and halting deforestation. A small remaining fraction of emissions can then be offset using carbon dioxide removal. People often use the terms ''net-zero emissions'', ''carbon neutrality,'' and ''climate neutrality'' with the same meaning. However, in some cases, these terms have different meanings. For example, some standards for ''carbon neutral certification'' allow a lot of carbon offsetting. But ''net zero standards'' require reducing emissions to more than 90% and then only offsetting the remaining 10% or less to fall in line with 1.5 °C targets. Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Resources Canada
Natural Resources Canada (NRCan; ; )Natural Resources Canada is the applied title under the Federal Identity Program; the legal title is Department of Natural Resources (). is the department of the Government of Canada responsible for natural resources, energy, minerals and metals, forests, earth sciences, mapping, and remote sensing. It was formed in 1994 by amalgamating the Department of Energy, Mines and Resources with the Department of Forestry. Under the ''Constitution Act, 1867'', primary responsibility for natural resources falls to provincial governments, however, the federal government has jurisdiction over off-shore resources, trade and commerce in natural resources, statistics, international relations, and boundaries. The department administers federal legislation relating to natural resources, including energy, forests, minerals and metals. The department also collaborates with American and Mexican government scientists, along with the Commission for Environmental ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |