|
Lanthanum Strontium Ferrite
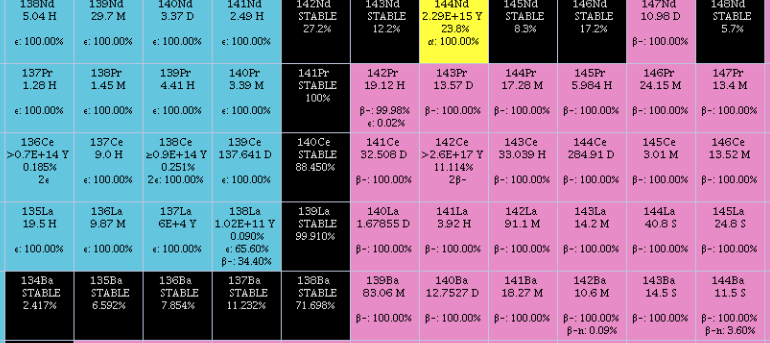
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is used by some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the ancient Greek (), meaning 'to lie hidden'. Although it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yttrium, Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements. Bastnäsite was first described by the Swedish chemist Wilhelm Hisinger in 1838. It is named for the Bastnäs mine near Riddarhyttan, Västmanland, Sweden. Bastnäsite also occurs as very high-quality specimens at the Zagi Mountains, Pakistan. Bastnäsite occurs in alkali granite and syenite and in associated pegmatites. It also occurs in carbonatites and in ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pergamon Press
Pergamon Press was an Oxford-based publishing house, founded by Paul Rosbaud and Robert Maxwell, that published scientific and medical books and journals. Originally called Butterworth-Springer, it is now an imprint of Elsevier. History The core company, Butterworth-Springer, started in 1948 to bring the "Springer know-how and techniques of aggressive publishing in science"Joe Haines (1988) ''Maxwell'', Houghton Mifflin, p. 137. to Britain. Paul Rosbaud was the man with the knowledge. When Maxwell acquired the company in 1951, Rosbaud held a one-quarter share. They changed the house name to Pergamon Press, using a logo that was a reproduction of a Greek coin from Pergamon. Maxwell and Rosbaud worked together growing the company until May 1956, when, according to Joe Haines, Rosbaud was sacked. When Pergamon Press started it had only six serials and two books. Initially the company headquarters was in Fitzroy Square in West End of London. In 1959, the company moved into He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pure And Applied Chemistry
''Pure and Applied Chemistry'' is the official journal for the International Union of Pure and Applied Chemistry (IUPAC). It is published monthly by Walter de Gruyter Walter de Gruyter GmbH, known as De Gruyter (), is a German scholarly publishing house specializing in academic literature. History The roots of the company go back to 1749 when Frederick the Great granted the Königliche Realschule in Be ... and contains recommendations and reports, and lectures from symposia. References Chemistry journals Academic journals established in 1960 De Gruyter academic journals {{Chemistry-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are barite ( barium sulfate, BaSO4) and witherite ( barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1772, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth Metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactivity (chemistry), reactive metals at standard temperature and pressure. Together with helium, these elements have in common an outer atomic orbital, s orbital which is full —that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with electric charge, charge +2, and an oxidation state of +2. Helium is grouped with the noble gases and not with the alkaline earth metals, but it is theorized to have some similarities to beryllium when forced into bonding and has sometimes been suggested to belong to group 2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney Failure
Kidney failure, also known as renal failure or end-stage renal disease (ESRD), is a medical condition in which the kidneys can no longer adequately filter waste products from the blood, functioning at less than 15% of normal levels. Kidney failure is classified as either acute kidney failure, which develops rapidly and may resolve; and chronic kidney failure, which develops slowly and can often be irreversible. Symptoms may include leg swelling, feeling tired, vomiting, loss of appetite, and confusion. Complications of acute and chronic failure include uremia, hyperkalemia, and volume overload. Complications of chronic failure also include heart disease, high blood pressure, and anaemia. Causes of acute kidney failure include low blood pressure, blockage of the urinary tract, certain medications, muscle breakdown, and hemolytic uremic syndrome. Causes of chronic kidney failure include diabetes, high blood pressure, nephrotic syndrome, and polycystic kidney diseas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperphosphatemia
Hyperphosphatemia is an electrolyte disorder in which there is an elevated level of phosphate in the blood. Most people have no symptoms while others develop calcium deposits in the soft tissue. The disorder is often accompanied by low calcium blood levels, which can result in muscle spasms. Causes include kidney failure, pseudohypoparathyroidism, hypoparathyroidism, diabetic ketoacidosis, tumor lysis syndrome, and rhabdomyolysis. Diagnosis is generally based on a blood phosphate level exceeding 1.46 mmol/L (4.5 mg/dL). Levels may appear falsely elevated with high blood lipid levels, high blood protein levels, or high blood bilirubin levels. Treatment may include a phosphate low diet and antacids like calcium carbonate that bind phosphate. Occasionally, intravenous normal saline or kidney dialysis may be used. How commonly it occurs is unclear. Signs and symptoms Signs and symptoms include ectopic calcification, secondary hyperparathyroidism, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Binder
Phosphate binders are medications used to reduce the absorption of dietary phosphate; they are taken along with meals and snacks. They are frequently used in people with chronic kidney failure (CKF), who are less able to excrete phosphate, resulting in an elevated serum phosphate. Mechanism of action These agents work by binding to phosphate in the GI tract, thereby making it unavailable to the body for absorption. Hence, these drugs are usually taken with meals to bind any phosphate that may be present in the ingested food. Phosphate binders may be simple molecular entities (such as magnesium, aluminium, calcium, or lanthanum salts) that react with phosphate and form an insoluble compound. Calcium carbonate Calcium-based phosphate binders, such as calcium carbonate, directly decrease phosphate levels by creating insoluble calcium–phosphate complexes which gets eliminated in the feces. Lanthanum carbonate Non-calcium-based phosphate binders, including lanthanum carbonate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Carbonate
Lanthanum carbonate, La( C O3)3, is the salt formed by lanthanum(III) cations and carbonate anions. It is an ore of lanthanum metal ( bastnäsite), along with monazite. Chemistry Lanthanum carbonate is used as a starting material in lanthanum chemistry, particularly in forming mixed oxides, for example * for production of lanthanum strontium manganite, primarily for solid oxide fuel cell applications; * for production of certain high-temperature superconductors, such as LaSrCuO. Medical uses Lanthanum carbonate is used in medicine as a phosphate binder. As a medication it is sold under the trade name Fosrenol by the pharmaceutical company Shire Pharmaceuticals. Due to its large size (1000 mg tablet is 2.2 cm in diameter), it may be possible to choke on the tablet if it is not chewed. It is prescribed for the treatment of hyperphosphatemia, primarily in patients with chronic kidney disease. It is taken with meals and binds to dietary phosphate, preventing phosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Tungsten Arc Welding
Gas tungsten arc welding (GTAW, also known as tungsten inert gas welding or TIG, tungsten argon gas welding or TAG, and heliarc welding when helium is used) is an arc welding process that uses a non-consumable tungsten electrode to produce the weld. The weld area and electrode are protected from oxidation or other atmospheric contamination by an inert shielding gas (argon or helium). A filler metal is normally used, though some welds, known as ' autogenous welds', or ' fusion welds' do not require it. A constant-current welding power supply produces electrical energy, which is conducted across the arc through a column of highly ionized gas and metal vapors known as a plasma. The process grants the operator greater control over the weld than competing processes such as shielded metal arc welding and gas metal arc welding, allowing stronger, higher-quality welds. However, TIG welding is comparatively more complex and difficult to master, and furthermore, it is significantly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scintillator
A scintillator ( ) is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed energy in the form of light). Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed (necessitating anywhere from a few nanoseconds to hours depending on the material). The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon. Principle of operation A scintillation detector or scintillation counter is obtained when a scintillator is coupled to an electronic light sensor such as a photomultiplier tube (PMT), photodiode, or silicon photomultiplier. PMTs absorb the light emitted by the scintillator and re-emit it in the form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |