|
JWH-116
JWH-116 is a synthetic cannabinoid receptor ligand from the naphthoylindole family. It is the indole 2-ethyl derivative of related compound JWH-018. The binding affinity of JWH-116 for the CB1 receptor is reported as Ki = 52 ± 5 nM. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as JWH-116 are Schedule I Controlled Substances. See also * JWH-018 * JWH-081 References JWH cannabinoids Naphthoylindoles Designer drugs CB1 receptor agonists {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH Cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
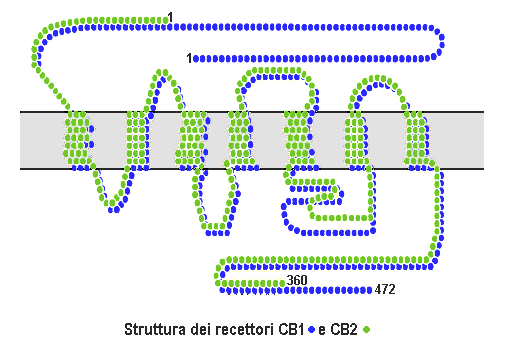
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoylindole
Naphthoylindoles are a class of synthetic cannabinoids Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids ( THC, CBD and many others) in cannabis plants attach. These novel psychoactive substances should not be confused with syntheti .... See also * Structural scheduling of synthetic cannabinoids References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends". As a full agonist at both the CB1 and CB2 cannabinoid receptors, this chemical compound is classified as an analgesic medication. The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis. These compounds work by mimicking the body's naturally-produced endocannabinoid hormones such as 2-AG and anandamide (AEA), which are biologically active and can exacerbate or inhibit nerve signaling. As the cause is poorly understood in chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schedule I Controlled Substance
This is the list of Schedule I drugs as defined by the United States Controlled Substances Act.21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to be placed in this schedule: # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. Except as specifically authorized, it is illegal for any person: # to manufacture, distribute, or dispense, or possess with intent to manufacture, distribute, or dispense, a controlled substance; or # to create, distribute, dispense, or possess with intent to distribute or dispense, a counterfeit substance. Additional substances are added to the list by the Secretary of Health and Human Services pursuant to 21 CFR Title 21 is the portion of the Code of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-081
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at approximately 10x the affinity at CB2(12.4nM). It was discovered by and named after John W. Huffman. JWH-081 may be neurotoxic to animals when administered in high doses. Legal status In the United States, JWH-081 is a Schedule I Controlled Substance. As of October 2015, JWH-081 is a controlled substance in China. See also * JWH-018 *JWH-098 JWH-098 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It is the indole 2-methyl derivative of a closely related compound JWH-081, but has markedly different affinity for the CB1 and CB2 receptors. While JWH-081 ... * JWH-164 * JWH-198 * JWH-210 References Designer drugs JWH cannabinoids Naphthoylindoles Phenol ethers CB1 receptor agonists {{ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoylindoles
Naphthoylindoles are a class of synthetic cannabinoids Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids ( THC, CBD and many others) in cannabis plants attach. These novel psychoactive substances should not be confused with syntheti .... See also * Structural scheduling of synthetic cannabinoids References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |