|
Isovaleryl-coenzyme A
Isovaleryl-CoA (also known as 3-methylbutyryl-CoA) is a metabolic intermediate formed during the catabolism of the branched-chain amino acid, Leucine. It is a short-chain acyl-CoA thioester that plays a key role in mitochondrial energy metabolism. The compound is converted into 3-Methylcrotonyl-CoA, 3-methylcrotonyl-CoA by the enzyme isovaleryl-CoA dehydrogenase (IVD), a flavoprotein that catalyzes the third step in the leucine degradation pathway. Deficiency of this enzyme activity results in the accumulation of isovaleryl-CoA and related metabolites, leading to a rare autosomal recessive disorder known as isovaleric acidemia, characterized by metabolic crises, developmental delays, and a distinctive odor due to isovaleric acid buildup. The metabolism of isovaleryl-CoA is vital for proper amino acid utilization and energy homeostasis in humans. Catalysis of Isovaleryl-CoA dehydrogenase The enzyme, Isovaleryl-CoA dehydrogenase (IVD), part of the family of Acyl-CoA dehydrogenase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FADH₂
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex. FAD can exist in four redox states, which are the flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone. FAD is converted between these states by accepting or donating electrons. FAD, in its fully oxidized form, or quinone form, accepts two electrons and two protons to become FADH2 (hydroquinone form). The semiquinone (FADH·) can be formed by either reduction of FAD or oxidation of FADH2 by accepting or donating one electron and one proton, respectively. Some proteins, however, generate and maintain a superoxidized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricarboxylic Acid
A tricarboxylic acid is an organic carboxylic acid that contain three carboxyl functional groups (−COOH). A well-known example is citric acid. Promient examples Some prominent substituted tricarboxylic acids Citric acid, is used in the citric acid cyclealso known as the ''tricarboxylic acid'' (''TCA'') ''cycle'' or ''Krebs cycle''which is fundamental to all aerobic organisms. Nitrilotriacetic acid (NTA) is a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+. See also * Citric acid cycle (tricarboxylic acid cycle) * Dicarboxylic acid In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarbox ... * Mellitic acid References Literature *{{cite journal , title = The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist. , author = Ryan J. Mailloux, Robin Bériaul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetic Acid
Acetoacetic acid ( IUPAC name: 3-oxobutanoic acid, also known as acetonecarboxylic acid or diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta- keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid. Biochemistry Under typical physiological conditions, acetoacetic acid exists as its conjugate base, acetoacetate: : Unbound acetoacetate is primarily produced by liver mitochondria from its thioester with coenzyme A (CoA): : The acetoacetyl-CoA itself is formed by three routes: *3-hydroxy-3-methylglutaryl CoA releases acetyl CoA and acetoacetate: *: *Acetoacetyl-CoA can come from beta oxidation of butyryl-CoA: *: *Condensation of pair of acetyl CoA molecules as catalyzed by thiolase. *: In mammals, acetoacetate produced in the liver (along with the other two " ketone bodies") is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a cysteamine, β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through Beta oxidation, β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Methylglutaconyl-CoA
3-Methylglutaconyl-CoA (MG-CoA), also known as β-methylglutaconyl-CoA, is an intermediate in the metabolism of leucine. It is metabolized into HMG-CoA β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate pathway, mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase .... Leucine metabolism See also * Methylcrotonyl-CoA carboxylase * Methylglutaconyl-CoA hydratase References Organophosphates Thioesters of coenzyme A {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
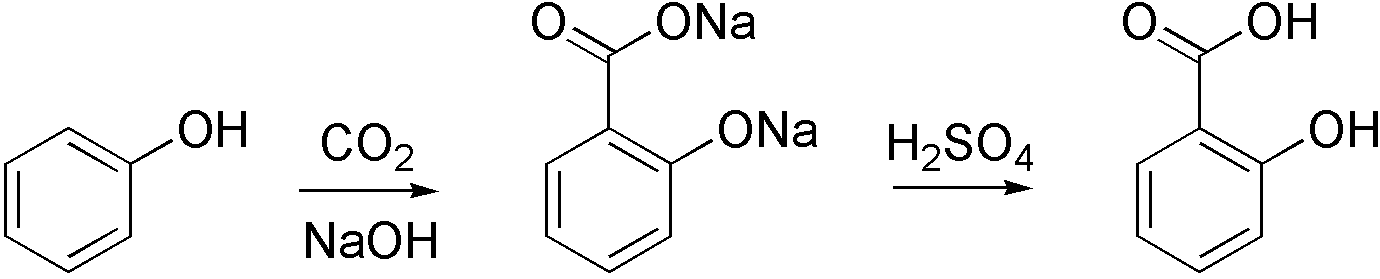
Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation (biochemistry), fermentation processes such as anaerobic glycolysis. The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle, producing carbon dioxide and the energetic reducing agent, electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to ATP synthase, produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a ser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inner Mitochondrial Membrane
The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space. Structure The structure of the inner mitochondrial membrane is extensively folded and compartmentalized. The numerous invaginations of the membrane are called cristae, separated by crista junctions from the inner boundary membrane juxtaposed to the outer membrane. Cristae significantly increase the total membrane surface area compared to a smooth inner membrane and thereby the available working space for oxidative phosphorylation. The inner membrane creates two compartments. The region between the inner and outer membrane, called the intermembrane space, is largely continuous with the cytosol, while the more sequestered space inside the inner membrane is called the matrix. Cristae For typical liver mitochondria, the area of the inner membrane is about 5 times as large as the outer membrane due to cristae. This ratio is variable and mitocho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration Gradient
Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. ''Fick's first law'': Movement of particles from high to low concentration (diffusive flux) is directly proportional to the particle's concentration gradient. ''Fick's second law'': Prediction of change in concentration gradient with time due to diffusion. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transport Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redox reactions are driven by the difference in the Gibbs free energy of reactants and products. The free energy released when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Adenine Dinucleotide
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex. FAD can exist in four redox states, which are the flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone. FAD is converted between these states by accepting or donating electrons. FAD, in its fully oxidized form, or quinone form, accepts two electrons and two protons to become FADH2 (hydroquinone form). The semiquinone (FADH·) can be formed by either reduction of FAD or oxidation of FADH2 by accepting or donating one electron and one proton, respectively. Some proteins, however, generate and maintain a super ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |