|
Isotopic Dilution
Isotope dilution analysis is a method of determining the quantity of chemical substances. In its most simple conception, the method of isotope dilution comprises the addition of known amounts of isotopically enriched substance to the analyzed sample. Mixing of the isotopic standard with the sample effectively "dilutes" the isotopic enrichment of the standard and this forms the basis for the isotope dilution method. Isotope dilution is classified as a method of internal standardisation, because the standard (isotopically enriched form of analyte) is added directly to the sample. In addition, unlike traditional analytical methods which rely on signal intensity, isotope dilution employs signal ratios. Owing to both of these advantages, the method of isotope dilution is regarded among chemistry measurement methods of the highest metrological standing. Isotopes are variants of a particular chemical element which differ in neutron number. All isotopes of a given element have the same num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principle Of Isotope Dilution
A principle may relate to a fundamental truth or proposition that serves as the foundation for a system of beliefs or behavior or a chain of reasoning. They provide a guide for behavior or evaluation. A principle can make values explicit, so they are expressed in the form of rules and standards. Principles unpack the values underlying them more concretely so that the values can be more easily operationalized in policy statements and actions. In law, higher order, overarching principles establish rules to be followed, modified by sentencing guidelines relating to context and proportionality. In science and nature, a principle may define the essential characteristics of the system, or reflect the system's designed purpose. The effective operation would be impossible if any one of the principles was to be ignored. A system may be explicitly based on and implemented from a document of principles as was done in IBM's 360/370 ''Principles of Operation''. It is important to differen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Volume
Blood volume (volemia) is the volume of blood ( blood cells and plasma) in the circulatory system of any individual. Humans A typical adult has a blood volume of approximately 5 liters, with females and males having approximately the same blood percentage by weight (approx 7 to 8%) Blood volume is regulated by the kidneys. Blood volume (BV) can be calculated given the hematocrit (HC; the fraction of blood that is red blood cells) and plasma volume (PV), with the hematocrit being regulated via the blood oxygen content regulator: :BV = \frac Blood volume measurement may be used in people with congestive heart failure, chronic hypertension, kidney failure and critical care. The use of relative blood volume changes during dialysis is of questionable utility. Total Blood Volume can be measured manually via the Dual Isotope or Dual Tracer Technique, a classic technique, available since the 1950s. This technique requires double labeling of the blood; that is 2 injections and 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Standard
In a chemical analysis, the internal standard method involves adding the same amount of a chemical substance to each sample and calibration solution. The internal standard responds proportionally to changes in the analyte and provides a similar, but not identical, measurement signal. It must also be absent from the sample matrix to ensure there is no other source of the internal standard present. Taking the ratio of analyte signal to internal standard signal and plotting it against the analyte concentrations in the calibration solutions will result in a calibration curve. The calibration curve can then be used to calculate the analyte concentration in an unknown sample. Selecting an appropriate internal standard accounts for random and systematic sources of uncertainty that arise during sample preparation or instrument fluctuation. This is because the ratio of analyte relative to the amount of internal standard is independent of these variations. If the measured value of the analyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
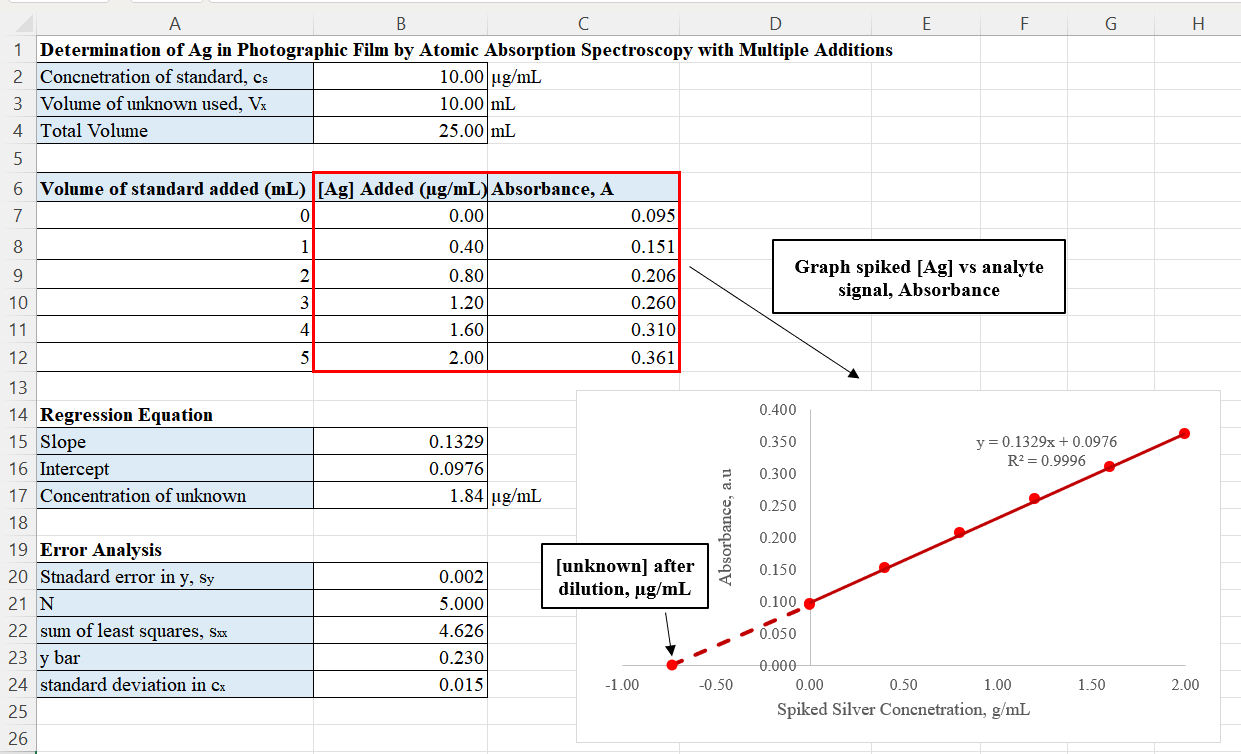
Standard Addition
The Standard addition method, also called known addition, often used in analytical chemistry, quantifies the analyte present in an unknown. This method is useful for analyzing complex samples where a matrix effect interferes with the analyte signal. In comparison to the calibration curve method, the standard addition method has the advantage of the matrices of the unknown and standards being nearly identical. This minimizes the potential bias arising from the matrix effect when determining the concentration. Variations Standard addition involves adding known amounts of analyte to an unknown sample, a process known as ''spiking''. By increasing the number of spikes, the analyst can extrapolate for the analyte concentration in the unknown that has not been spiked. There are multiple approaches to the standard addition. The following section summarize each approach. Single standard addition used in polarography In classic polarography, the standard addition method involves creati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytica Chimica Acta
''Analytica Chimica Acta'' is a peer-reviewed scientific journal published since 1947 that covers original research and reviews of fundamental and applied aspects of analytical chemistry Analytical skill, Analytical chemistry studies and uses instruments and methods to Separation process, separate, identify, and Quantification (science), quantify matter. In practice, separation, identification or quantification may constitute t .... The editors-in-chief are Prof. Lutgarde Buydens and Prof. James Landers. See also * List of scientific journals in chemistry * Analytical chemistry * Chemistry References Elsevier academic journals Chemistry journals Academic journals established in 1947 Weekly journals English-language journals 1947 establishments in the Netherlands {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Padé Approximant
In mathematics, a Padé approximant is the "best" approximation of a function near a specific point by a rational function of given order. Under this technique, the approximant's power series agrees with the power series of the function it is approximating. The technique was developed around 1890 by Henri Padé, but goes back to Georg Frobenius, who introduced the idea and investigated the features of rational approximations of power series. The Padé approximant often gives better approximation of the function than truncating its Taylor series, and it may still work where the Taylor series does not converge. For these reasons Padé approximants are used extensively in computer calculations. They have also been used as auxiliary functions in Diophantine approximation and transcendental number theory, though for sharp results ad hoc methods—in some sense inspired by the Padé theory—typically replace them. Since a Padé approximant is a rational function, an artificial sin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Journal Of Mass Spectrometry
The ''International Journal of Mass Spectrometry'' is a monthly peer-reviewed scientific journal covering all aspects of mass spectrometry, including instrumentation and applications in biology, chemistry, geology, and physics. It was established in 1968 as the ''International Journal of Mass Spectrometry and Ion Physics'' and was renamed ''International Journal of Mass Spectrometry and Ion Processes'' in 1983, before obtaining its current title in 1998. It is published by Elsevier and the editors-in-chief are Julia Laskin (Purdue University) and Zheng Ouyang (Tsinghua University). Abstracting and indexing The journal is abstracted and indexed in: *Chemical Abstracts Service *Current Contents/Physical, Chemical & Earth Sciences * EBSCO databases * Embase *Food Science and Technology Abstracts *FRANCIS *Inspec * PASCAL *Science Citation Index Expanded *Scopus According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrochimica Acta B
''Spectrochimica Acta Part B: Atomic Spectroscopy'' is a monthly peer-reviewed scientific journal covering spectroscopy. The journal was established in 1939 as ''Spectrochimica Acta''. In 1967, ''Spectrochimica Acta'' was split into two journals, '' Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy'' and ''Spectrochimica Acta Part B: Atomic Spectroscopy''. Part B obtained its current title around the time of the split. According to the ''Journal Citation Reports'', the journal has a 2019 impact factor of 3.086. the editor-in-chief An editor-in-chief (EIC), also known as lead editor or chief editor, is a publication's editorial leader who has final responsibility for its operations and policies. The editor-in-chief heads all departments of the organization and is held accoun ... is Alessandro De Giacomo of the University of Bari, Italy. See also * Elsevier / Spectrochimica Acta Atomic Spectroscopy Award References External links * Spectroch. Acta B at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accreditation And Quality Assurance
Accreditation is the independent, third-party evaluation of a conformity assessment body (such as certification body, inspection body or laboratory) against recognised standards, conveying formal demonstration of its impartiality and competence to carry out specific conformity assessment tasks (such as certification, inspection and testing). Accreditation bodies are established in many economies with the primary purpose of ensuring that conformity assessment bodies are subject to oversight by an authoritative body. Accreditation bodies, that have been peer evaluated as competent, sign regional and international arrangements to demonstrate their competence. These accreditation bodies then assess and accredit conformity assessment bodies to the relevant standards. An authoritative body that performs accreditation is called an 'accreditation body'. The International Accreditation Forum (IAF) and International Laboratory Accreditation Cooperation (ILAC) provide international recognitio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical And Bioanalytical Chemistry
Analytic or analytical may refer to: Chemistry * Analytical chemistry, the analysis of material samples to learn their chemical composition and structure * Analytical technique, a method that is used to determine the concentration of a chemical compound or chemical element * Analytical concentration Mathematics * Abstract analytic number theory, the application of ideas and techniques from analytic number theory to other mathematical fields * Analytic combinatorics, a branch of combinatorics that describes combinatorial classes using generating functions * Analytic element method, a numerical method used to solve partial differential equations * Analytic expression or analytic solution, a mathematical expression using well-known operations that lend themselves readily to calculation * Analytic geometry, the study of geometry based on numerical coordinates rather than axioms * Analytic number theory, a branch of number theory that uses methods from mathematical analysis Mathe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Mean
In mathematics, the geometric mean is a mean or average which indicates a central tendency of a finite collection of positive real numbers by using the product of their values (as opposed to the arithmetic mean which uses their sum). The geometric mean of numbers is the Nth root, th root of their product (mathematics), product, i.e., for a collection of numbers , the geometric mean is defined as : \sqrt[n]. When the collection of numbers and their geometric mean are plotted in logarithmic scale, the geometric mean is transformed into an arithmetic mean, so the geometric mean can equivalently be calculated by taking the natural logarithm of each number, finding the arithmetic mean of the logarithms, and then returning the result to linear scale using the exponential function , :\sqrt[n] = \exp \left( \frac \right). The geometric mean of two numbers is the square root of their product, for example with numbers and the geometric mean is \textstyle \sqrt = The geometric mean o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |