|
Isotopes Of Bromine
Bromine (35Br) has two stable isotopes, 79Br and 81Br, and 35 known radioisotopes, the most stable of which is 77Br, with a half-life of 57.036 hours. Like the radioactive isotopes of iodine, radioisotopes of bromine, collectively radiobromine, can be used to label biomolecules for nuclear medicine; for example, the positron emitters 75Br and 76Br can be used for positron emission tomography. Radiobromine has the advantage that organobromides are more stable than analogous organoiodides, and that it is not uptaken by the thyroid like iodine. List of isotopes , -id=Bromine-68 , 68Br , style="text-align:right" , 35 , style="text-align:right" , 33 , 67.95836(28)# , ~35 ns , p? , 67Se , 3+# , , , -id=Bromine-69 , 69Br , style="text-align:right" , 35 , style="text-align:right" , 34 , 68.950338(45) , 99.4%) , 76Br , rowspan=2, (4)+ , rowspan=2, , rowspan=2, , - , β+ (99.99%) , 78Se , rowspan=2, 1+ , rowspan=2, , rowspan=2, , - , β− (<0.0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium-75
Selenium has six natural isotopes that occur in significant quantities, along with the trace isotope 79Se, which occurs in minute quantities in uranium ores. Five of these isotopes are stable: 74Se, 76Se, 77Se, 78Se, and 80Se. The last three also occur as fission products, along with 79Se, which has a half-life of 327,000 years,The half-life of 79Se and 82Se, which has a very long half-life (~1020 years, decaying via to 82Kr) and for practical purposes can be considered to be stable. There are 23 other unstable isotopes that have been c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Arsenic
Arsenic (33As) has 32 known isotopes and at least 10 isomers. Only one of these isotopes, 75As, is stable; as such, it is considered a monoisotopic element. The longest-lived radioisotope is 73As with a half-life of 80 days. List of isotopes , -id=Arsenic-64 , rowspan=2 , 64As , rowspan=2 style="text-align:right" , 33 , rowspan=2 style="text-align:right" , 31 , rowspan=2 , 63.95756(22)# , rowspan=2 , 69.0(14) ms , β+ , 64Ge , rowspan=2 , 0+# , rowspan=2 , , - , β+, p? , 63Ga , -id=Arsenic-65 , rowspan=2 , 65As , rowspan=2 style="text-align:right" , 33 , rowspan=2 style="text-align:right" , 32 , rowspan=2 , 64.949611(91) , rowspan=2 , 130.3(6) ms , β+ , 65Ge , rowspan=2 , 3/2−# , rowspan=2 , , - , β+, p? , 64Ga , -id=Arsenic-66 , 66As , style="text-align:right" , 33 , style="text-align:right" , 33 , 65.9441488(61) , 95.77(23) ms , β+ , 66Ge , 0+ , , -id=Arsenic-66m1 , style="text-indent:1em" , 66m1As , colspan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Selenium
Selenium has six natural isotopes that occur in significant quantities, along with the trace isotope 79Se, which occurs in minute quantities in uranium ores. Five of these isotopes are stable: 74Se, 76Se, 77Se, 78Se, and 80Se. The last three also occur as fission products, along with 79Se, which has a half-life of 327,000 years,The half-life of 79Se and 82Se, which has a very long half-life (~1020 years, decaying via to 82Kr) and for practical purposes can be considered to be stable. There are 23 other unstable isotopes that have been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Krypton
There are 34 known isotopes of krypton (36Kr) with atomic mass numbers from 67 to 103. Naturally occurring krypton is made of five stable isotopes and one () which is slightly radioactive with an extremely long half-life, plus traces of radioisotopes that are produced by cosmic rays in the atmosphere. List of isotopes , -id=Krypton-67 , rowspan=2, 67Kr , rowspan=2 style="text-align:right" , 36 , rowspan=2 style="text-align:right" , 31 , rowspan=2, 66.98331(46)# , rowspan=2, 7.4(29) ms , β+? (63%) , 67Br , rowspan=2, 3/2-# , rowspan=2, , rowspan=2, , - , 2p (37%) , 65Se , -id=Krypton-68 , rowspan=3, 68Kr , rowspan=3 style="text-align:right" , 36 , rowspan=3 style="text-align:right" , 32 , rowspan=3, 67.97249(54)# , rowspan=3, 21.6(33) ms , β+, p (>90%) , 67Se , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+? (98.7%) , 70Br , rowspan=2, 0+ , rowspan=2, , rowspan=2, , - , β+, p (<1.3%) , 69Se , -id=Krypton-71 , rows ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
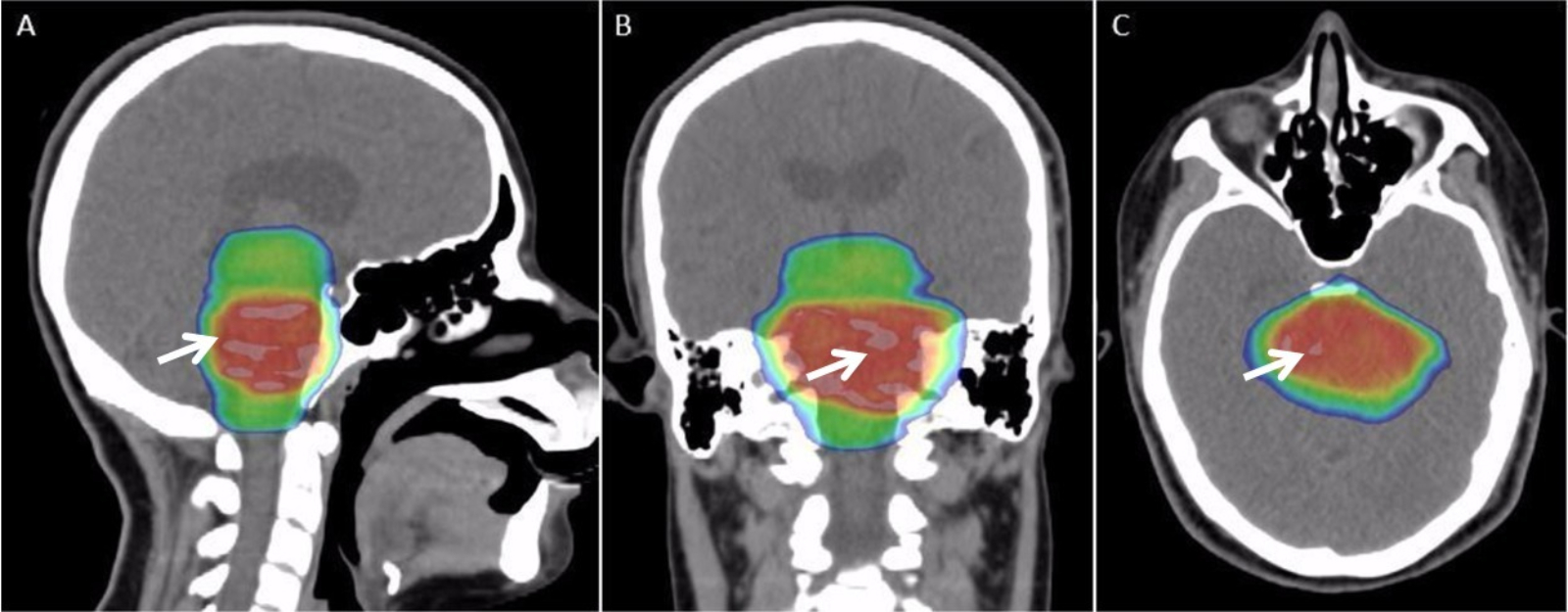
Radiotherapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Auger Electron
The Auger effect (; ) or Meitner-Auger effect is a physical phenomenon in which atoms eject electrons. It occurs when an inner-shell vacancy in an atom is filled by an electron, releasing energy that causes the emission of another electron from a different shell of the same atom. When a core electron is removed, leaving a vacancy, an electron from a higher energy level may fall into the vacancy, resulting in a release of energy. For light atoms (Z<12), this energy is most often transferred to a valence electron which is subsequently ejected from the atom. This second ejected electron is called an Auger electron. For heavier atomic nuclei, the release of the energy in the form of an emitted becomes gradually more probable. Effect Upon ejection, the |
Annihilation Radiation
Annihilation radiation is a term used in Gamma spectroscopy for the photon radiation produced when a particle and its antiparticle collide and annihilate. Most commonly, this refers to 511-k eV photons produced by an electron interacting with a positron. These photons are frequently referred to as gamma rays, despite having their origin outside the nucleus, due to unclear distinctions between types of photon radiation. The positively-charged antiparticle of the electron (known as the positron) is emitted from the nucleus as it undergoes β+ decay. The positron travels a short distance (a few millimeters), depositing any excess energy before it combines with a free electron. The mass of the e- and e+ is completely converted into two photons with an energy of 511 keV each. These annihilation photons are emitted in opposite directions, 180˚ apart. This is the basis for PET scanners in a process called coincidence counting. Annihilation radiation is not monoenergetic, unlike gamma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collimator
A collimator is a device which narrows a beam of particles or waves. To narrow can mean either to cause the directions of motion to become more aligned in a specific direction (i.e., make collimated light or parallel rays), or to cause the spatial cross section of the beam to become smaller (beam limiting device). History The English physicist Henry Kater was the inventor of the floating collimator, which rendered a great service to practical astronomy. He reported about his invention in January 1825. In his report, Kater mentioned previous work in this area by Carl Friedrich Gauss and Friedrich Bessel. Optical collimators In optics, a collimator may consist of a curved mirror or lens with some type of light source and/or an image at its focus. This can be used to replicate a target focused at infinity with little or no parallax. In lighting, collimators are typically designed using the principles of nonimaging optics. Optical collimators can be used to calibrate other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
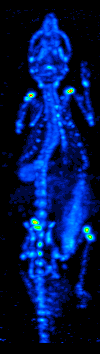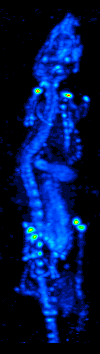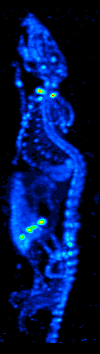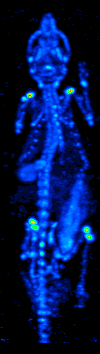
SPECT
Single-photon emission computed tomography (SPECT, or less commonly, SPET) is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera (that is, scintigraphy), but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required. The technique needs delivery of a gamma-emitting radioisotope (a radionuclide) into the patient, normally through injection into the bloodstream. On occasion, the radioisotope is a simple soluble dissolved ion, such as an isotope of gallium(III). Usually, however, a marker radioisotope is attached to a specific ligand to create a radioligand, whose properties bind it to certain types of tissues. This marriage allows the combination of ligand and radiopharmaceutical to be carried and bound to a place of interest in the body, where th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium-76
Selenium has six natural isotopes that occur in significant quantities, along with the trace isotope 79Se, which occurs in minute quantities in uranium ores. Five of these isotopes are stable: 74Se, 76Se, 77Se, 78Se, and 80Se. The last three also occur as fission products, along with 79Se, which has a half-life of 327,000 years,The half-life of 79Se and 82Se, which has a very long half-life (~1020 years, decaying via to 82Kr) and for practical purposes can be considered to be stable. There are 23 other unstable isotopes that have been c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisintegration, photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous fission, spontaneous and nuclear fission, induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |