|
Isotopes Of Argon
Argon (Ar) has 26 known isotopes, from Ar to Ar, of which three are stable (Ar, Ar, and Ar). On Earth, Ar makes up 99.6% of natural argon. The longest-lived radioactive isotopes are Ar with a half-life of 268 years, Ar with a half-life of 32.9 years, and Ar with a half-life of 35.04 days. All other isotopes have half-lives of less than two hours, and most less than one minute. The naturally occurring K, with a half-life of 1.248 years, decays to stable Ar by electron capture (10.72%) and by positron emission (0.001%), and also to stable Ca via beta decay (89.28%). These properties and ratios are used to determine the age of rocks through potassium–argon dating. Despite the trapping of Ar in many rocks, it can be released by melting, grinding, and diffusion. Almost all argon in the Earth's atmosphere is the product of K decay, since 99.6% of Earth's atmospheric argon is Ar, whereas in the Sun and presumably in primordial star-forming clouds, argon consists of K or alpha emi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmogenic Isotope
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom (see cosmic ray spallation). These nuclides are produced within Earth materials such as rocks or soil, in Earth's atmosphere, and in extraterrestrial items such as meteoroids. By measuring cosmogenic nuclides, scientists are able to gain insight into a range of geological and astronomical processes. There are both radioactive and stable cosmogenic nuclides. Some of these radionuclides are tritium, carbon-14 and phosphorus-32. Certain light (low atomic number) primordial nuclides (isotopes of lithium, beryllium and boron) are thought to have been created not only during the Big Bang, but also (and perhaps primarily) to have been made after the Big Bang, but before the condensation of the Solar System, by the process of cosmic ray s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying nuclear isomer, isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative (Sp < 0)—the proton is therefore unbound, and quantum tunneling, tunnels out of the nucleus in a finite time. The rate of proton emission is governed by the nuclear, Coulomb, and centrifugal potentials of the nucleus, where centrifugal potential affects a large part of the rate of proton emission. The half-life of a nucleus with respect to proton emission is affected by the proton energy and its orbital angular momentum. Proton emiss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Testing
Nuclear weapons tests are experiments carried out to determine the performance of nuclear weapons and the effects of Nuclear explosion, their explosion. Nuclear testing is a sensitive political issue. Governments have often performed tests to signal strength. Because of their destruction and fallout, testing has seen opposition by civilians as well as governments, with international bans having been agreed on. Thousands of tests have been performed, with most in the second half of the 20th century. The first nuclear device was detonated as a test by the United States at the Trinity site in New Mexico on July 16, 1945, with a yield approximately TNT equivalent, equivalent to 20 kilotons of TNT. The first thermonuclear weapon technology test of an engineered device, codenamed Ivy Mike, was tested at the Enewetak Atoll in the Marshall Islands on November 1, 1952 (local date), also by the United States. The largest nuclear weapon ever tested was the Tsar Bomba of the Soviet Union at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produced in different ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+ or 2+ indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. When produced in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV and a velocity in the vicinity of 4% of the speed of light. They are a highly ionizing form of particle radiation, with low penetration depth (stopped b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
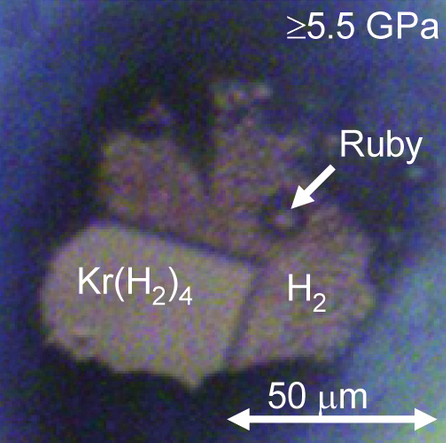
Noble Gas Compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 8 or 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton ( ionisation energy 14.0 eV), xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of or lower, in supersonic jets of noble gas, or under extremely high pressures with metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Science (journal)
''Science'' is the peer review, peer-reviewed academic journal of the American Association for the Advancement of Science (AAAS) and one of the world's top academic journals. It was first published in 1880, is currently circulated weekly and has a subscriber base of around 130,000. Because institutional subscriptions and online access serve a larger audience, its estimated readership is over 400,000 people. ''Science'' is based in Washington, D.C., United States, with a second office in Cambridge, UK. Contents The major focus of the journal is publishing important original scientific research and research reviews, but ''Science'' also publishes science-related news, opinions on science policy and other matters of interest to scientists and others who are concerned with the wide implications of science and technology. Unlike most scientific journals, which focus on a specific field, ''Science'' and its rival ''Nature (journal), Nature'' cover the full range of List of academ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The New York Times
''The New York Times'' (''NYT'') is an American daily newspaper based in New York City. ''The New York Times'' covers domestic, national, and international news, and publishes opinion pieces, investigative reports, and reviews. As one of the longest-running newspapers in the United States, the ''Times'' serves as one of the country's Newspaper of record, newspapers of record. , ''The New York Times'' had 9.13 million total and 8.83 million online subscribers, both by significant margins the List of newspapers in the United States, highest numbers for any newspaper in the United States; the total also included 296,330 print subscribers, making the ''Times'' the second-largest newspaper by print circulation in the United States, following ''The Wall Street Journal'', also based in New York City. ''The New York Times'' is published by the New York Times Company; since 1896, the company has been chaired by the Ochs-Sulzberger family, whose current chairman and the paper's publ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supernova Remnant
A supernova remnant (SNR) is the structure resulting from the explosion of a star in a supernova. The supernova remnant is bounded by an expanding shock wave, and consists of ejected material expanding from the explosion, and the interstellar material it sweeps up and shocks along the way. There are two common routes to a supernova: either a massive star may run out of fuel, ceasing to generate fusion energy in its core, and collapsing inward under the force of its own gravity to form a neutron star or a black hole; or a white dwarf star may accrete material from a companion star until it reaches a critical mass and undergoes a thermonuclear explosion. In either case, the resulting supernova explosion expels much or all of the stellar material with velocities as much as 10% the speed of light (or approximately 30,000 km/s) and a strong shock wave forms ahead of the ejecta. That heats the upstream plasma up to temperatures well above millions of K. The shock continuou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crab Nebula
The Crab Nebula (catalogue designations M1, NGC 1952, Taurus A) is a supernova remnant and pulsar wind nebula in the constellation of Taurus (constellation), Taurus. The common name comes from a drawing that somewhat resembled a crab with arms produced by William Parsons, 3rd Earl of Rosse, in 1842 or 1843 using a telescope. The nebula was discovered by English astronomer John Bevis in 1731. It corresponds with SN 1054, a bright supernova observed in 1054 C.E. by Native American, Japanese, and Arabic stargazers; this supernova was also recorded by Chinese astronomy, Chinese astronomers as a Guest star (astronomy), guest star. The nebula was the first astronomical object identified that corresponds with a historically-observed supernova explosion. At an apparent magnitude of 8.4, comparable to that of Titan (moon), Saturn's moon Titan, it is not visible to the naked eye but can be made out using binoculars under favourable conditions. The nebula lies in the Perseus Arm of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon Hydride
Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space. Properties Argonium is isoelectronic with hydrogen chloride. Its dipole moment is 2.18 D for the ground state. The binding energy is 369 kJ mol−1 (3.9 eV). This is smaller than that of and many other protonated species, but more than that of . Rotationless radiative lifetimes of different vibrational states vary with isotope and become shorter for the more rapid high-energy vibrations: : The force constant in the bond is calculated at 3.88 mdyne/Å2. Reactions *ArH+ + H2 → Ar + *ArH+ + C → Ar + CH+ *ArH+ + N → Ar + NH+ *ArH+ + O → Ar + OH+ *ArH+ + CO → Ar + COH+ But the reverse reaction happens: *Ar + → ArH+ + H. *Ar + → *ArH+ + H2 Ar+ + H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |