|
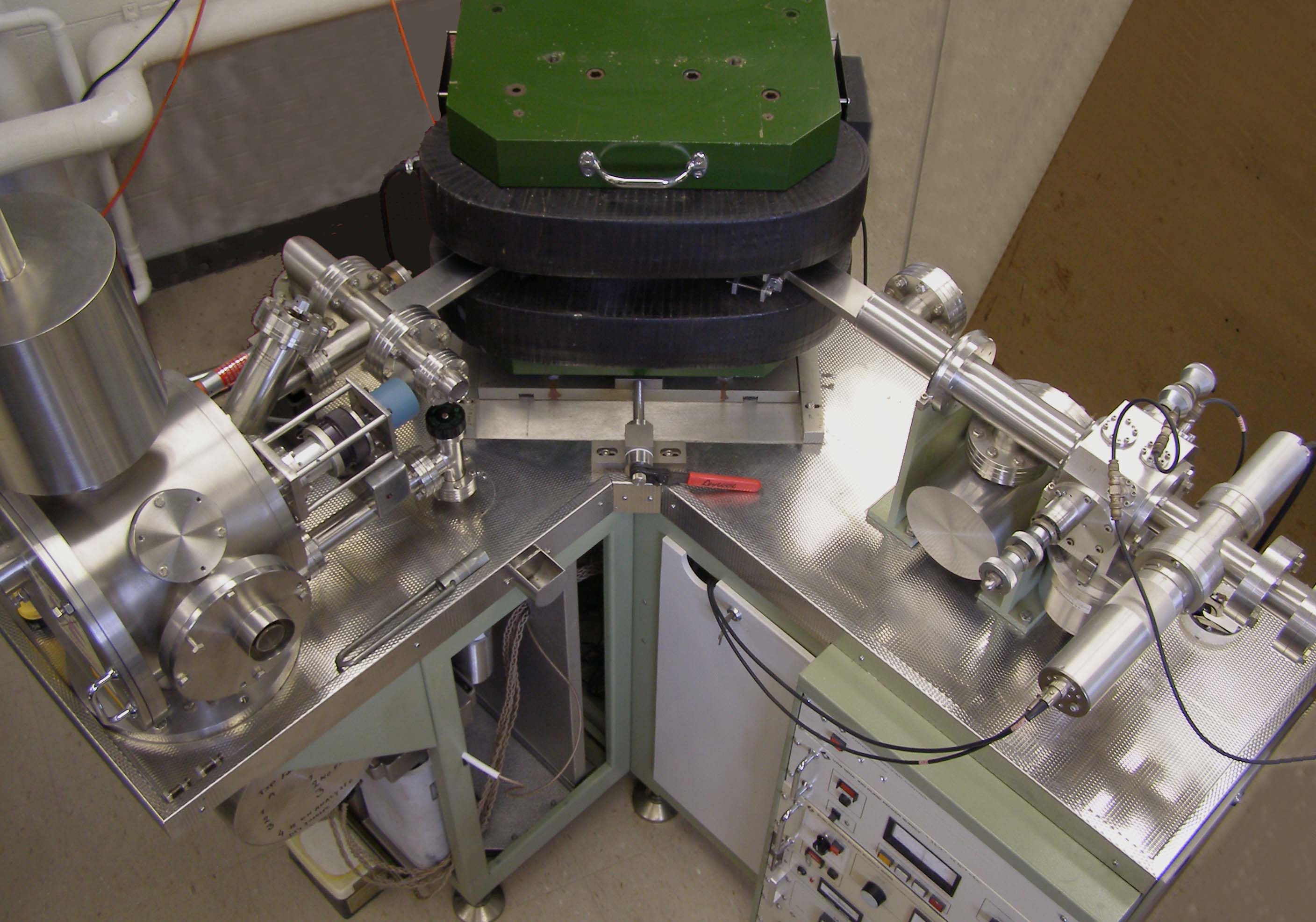
Isotope-ratio Mass Spectrometry
Isotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample. This technique has two different applications in the earth and environmental sciences. The analysis of ' stable isotopes' is normally concerned with measuring isotopic variations arising from mass-dependent isotopic fractionation in natural systems. On the other hand, radiogenic isotope analysis involves measuring the abundances of decay-products of natural radioactivity, and is used in most long-lived radiometric dating methods. Introduction The isotope-ratio mass spectrometer (IRMS) allows the precise measurement of mixtures of naturally occurring isotopes. Most instruments used for precise determination of isotope ratios are of the magnetic sector type. This type of analyzer is superior to the quadrupole type in this field of research for two reasons. First, it can be set up for multip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Ionization
Thermal ionization, also known as surface ionization or contact ionization, is a physical process whereby the atoms are desorption, desorbed from a hot surface, and in the process are ionized. Thermal ionization is used to make simple ion sources, for mass spectrometry and for generating ion beams. Thermal ionization has seen extensive use in determining atomic weights, in addition to being used in many geological/nuclear applications. Physics The likelihood of ionization is a function of the filament temperature, the work function of the filament substrate and the ionization energy of the chemical element, element. This is summarised in the Saha ionization equation, Saha–Langmuir equation: : \frac = \frac \exp \Bigg(\frac\Bigg) where :: \frac = ratio of ion number density to neutral number density :: \frac = ratio of statistical weights (degeneracy) of ionic (''g''+) and neutral (''g''0) states :: W = work function of surface :: \Delta E_\text = ionization energy of deso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium–strontium Dating
The rubidium–strontium dating method (Rb–Sr) is a radiometric dating technique, used by scientists to determine the age of rocks and minerals from their content of specific isotopes of rubidium (87Rb) and strontium (87Sr, 86Sr). One of the two naturally occurring isotopes of rubidium, 87Rb, decays to 87Sr with a half-life of 49.23 billion years. The radiogenic daughter, 87Sr, produced in this decay process is the only one of the four naturally occurring strontium isotopes that was not produced exclusively by stellar nucleosynthesis predating the formation of the Solar System. Over time, decay of 87Rb increases the amount of radiogenic 87Sr while the amount of other Sr isotopes remains unchanged. The ratio 87Sr/86Sr in a mineral sample can be accurately measured using a mass spectrometer. If the amount of Sr and Rb isotopes in the sample when it formed can be determined, the age can be calculated from the increase in 87Sr/86Sr. Different minerals that crystallized from the sam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Ionization Mass Spectrometry
Thermal ionization mass spectrometry (TIMS), also known as surface ionization, is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion beams based on the ion's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon–argon Dating
Argon–argon (or 40Ar/39Ar) dating is a radiometric dating method invented to supersede Potassium-argon dating, potassiumargon (K/Ar) dating in accuracy. The older method required splitting samples into two for separate potassium and argon measurements, while the newer method requires only one rock fragment or mineral grain and uses a single measurement of isotopes of argon, argon isotopes. 40Ar/39Ar dating relies on neutron irradiation from a nuclear reactor to convert a stable form of potassium (39K) into the radioactive 39Ar. As long as a standard of known age is co-irradiated with unknown samples, it is possible to use a single measurement of argon isotopes to calculate the 40K/40Ar* ratio, and thus to calculate the age of the unknown sample. 40Ar* refers to the radiogenic 40Ar, i.e. the 40Ar produced from radioactive decay of 40K. 40Ar* does not include atmospheric argon adsorbed to the surface or inherited through diffusion and its calculated value is derived from measuring t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various Chemical element, elements. Variations in isotopic abundance are measured by isotope-ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic fractionation, kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (Per mille, ‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a Reference Materials fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noble Gases
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some cases, oganesson (Og). Under standard conditions, the first six of these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points. The properties of oganesson are uncertain. The intermolecular force between noble gas atoms is the very weak London dispersion force, so their boiling points are all cryogenic, below . The noble gases' inertness, or tendency not to react with other chemical substances, results from their electron configuration: their outer shell of valence electrons is "full", giving them little tendency to participate in chemical reactions. Only a few hundred noble gas compounds are known to exist. The inertness of noble gases makes them useful whenever chemical reactions are un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of metals refining and the burning of Sour gas, sulfur-Sour crude oil, bearing fossil fuels. Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more. It was known to medieval alchemy, alchemists as "volatile spirit of sulfur". Structure and bonding SO2 is a bent molecule with ''C''2v Point groups in three dimensions, symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance (chemistry), resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Ratio Ms
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
South Carolina
South Carolina ( ) is a U.S. state, state in the Southeastern United States, Southeastern region of the United States. It borders North Carolina to the north and northeast, the Atlantic Ocean to the southeast, and Georgia (U.S. state), Georgia to the west and south across the Savannah River. Along with North Carolina, it makes up the Carolinas region of the East Coast of the United States, East Coast. South Carolina is the List of U.S. states and territories by area, 11th-smallest and List of U.S. states and territories by population, 23rd-most populous U.S. state with a recorded population of 5,118,425 according to the 2020 United States census, 2020 census. In , its GDP was $213.45 billion. South Carolina is composed of List of counties in South Carolina, 46 counties. The capital is Columbia, South Carolina, Columbia with a population of 136,632 in 2020; while its List of municipalities in South Carolina, most populous city is Charleston, South Carolina, Charleston with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peedee Formation
The Peedee Formation is a Formation (geology), geologic formation in North Carolina, North and South Carolina. A marine deposit representing an inner Neritic zone, neritic environment, named for exposures along the Great Peedee River, it preserves invertebrate (primarily Belemnitida, belemnites, Echinoderm, echinoderms and foraminifera) and vertebrate (primarily shark teeth, with some marine reptile remains) fossils dating to the Late Cretaceous (Maastrichtian). The formation is notable for its occurrence of ''Belemnitella americana'', known as the Pee Dee Belemnite (PDB), a long-standing standard in Delta13c, stable carbon isotope research. A single pterosaur femur, possibly an Azhdarchidae, Azhdarchid, from the Peedee formation is one of the few pterosaur body fossils found in Appalachia (landmass), Eastern North America. The stratigraphy of the formation spans from the early Maastrichtian (in South Carolina) to the late Maastrichtian shortly before the Cretaceous–Paleogene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |