|
Isenthalpic
An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, ''H''; or specific enthalpy, ''h''. Overview If a steady-state, steady-flow process is analysed using a control volume, everything outside the control volume is considered to be the ''surroundings''.G. J. Van Wylen and R. E. Sonntag, ''Fundamentals of Classical Thermodynamics'', Section 2.1 (3rd edition). Such a process will be isenthalpic if there is no transfer of heat to or from the surroundings, no work done on or by the surroundings, and no change in the kinetic energy of the fluid.G. J. Van Wylen and R. E. Sonntag, ''Fundamentals of Classical Thermodynamics'', Section 5.13 (3rd edition). This is a sufficient but not necessary condition for isoenthalpy. The necessary condition for a process to be isoenthalpic is that the sum of each of the terms of the energy balance other than enthalpy (work, heat, changes in kinetic energy, etc.) cancel each other, so that the enthalpy r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas Laws
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
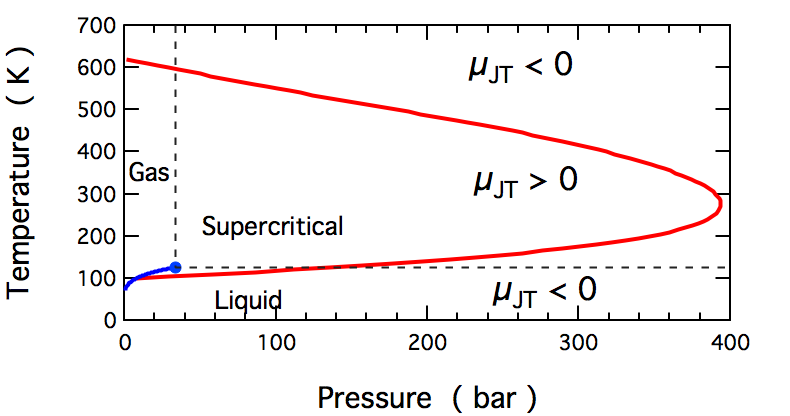
Joule–Thomson Effect
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a ''real'' gas or liquid (as differentiated from an ideal gas) when it is forced through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This procedure is called a ''throttling process'' or ''Joule–Thomson process''. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases experience the same effect but only at lower temperatures. Most liquids such as hydraulic oils will be warmed by the Joule–Thomson throttling process. The gas-cooling throttling process is commonly exploited in refrigeration processes such as liquefiers in air separation industrial process. In hydraulics, the warming effect from Joule–Thomson throttling can be used to find internall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isentropic Process
In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such. The word "isentropic" can be interpreted in another way, since its meaning is deducible from its etymology. It means a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Processes
Classical thermodynamics considers three main kinds of thermodynamic process: (1) changes in a system, (2) cycles in a system, and (3) flow processes. (1)A Thermodynamic process is a process in which the thermodynamic state of a system is changed. A change in a system is defined by a passage from an initial to a final state of thermodynamic equilibrium. In classical thermodynamics, the actual course of the process is not the primary concern, and often is ignored. A state of thermodynamic equilibrium endures unchangingly unless it is interrupted by a thermodynamic operation that initiates a thermodynamic process. The equilibrium states are each respectively fully specified by a suitable set of thermodynamic state variables, that depend only on the current state of the system, not on the path taken by the processes that produce the state. In general, during the actual course of a thermodynamic process, the system may pass through physical states which are not describable as thermody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces. A state of a thermodynamic system can be fully described in several different ways, by several different sets of thermodynamic state variables. A widely used distinction is between ''isolated'', ''closed'', and ''open'' thermodynamic systems. An isolated thermodynamic system has walls that are non-conductive of heat and perfectly reflective of all radiation, that are rigid and immovable, and that are impermeable to all forms of matter and all forces. (Some writers use the word 'closed' when here the word 'isolated' is being used.) A closed thermodynamic system is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |