|
Ischemic Cascade
The ischemic (ischaemic) cascade is a series of biochemical reactions that are initiated in the brain and other aerobic tissues after seconds to minutes of ischemia (inadequate blood supply). This is typically secondary to stroke, injury, or cardiac arrest due to heart attack. Most ischemic neurons that die do so due to the activation of chemicals produced during and after ischemia.Stroke Center of the Washington University School of Medicine. The ischemic cascade usually goes on for two to three hours but can last for days, even after normal blood flow returns. __TOC__ Mechanism A is a series of events in which one event triggers the next, in a ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brain
The brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head (cephalization), usually near organs for special senses such as visual perception, vision, hearing, and olfaction. Being the most specialized organ, it is responsible for receiving information from the sensory nervous system, processing that information (thought, cognition, and intelligence) and the coordination of motor control (muscle activity and endocrine system). While invertebrate brains arise from paired segmental ganglia (each of which is only responsible for the respective segmentation (biology), body segment) of the ventral nerve cord, vertebrate brains develop axially from the midline dorsal nerve cord as a brain vesicle, vesicular enlargement at the rostral (anatomical term), rostral end of the neural tube, with centralized control over all body segments. All vertebr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the optical rotation, dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxylic acid, carboxyl groups −COOH and one amine, amino group � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biology), morphology) and death. These changes include Bleb (cell biology), blebbing, Plasmolysis, cell shrinkage, Karyorrhexis, nuclear fragmentation, Pyknosis, chromatin condensation, Apoptotic DNA fragmentation, DNA fragmentation, and mRNA decay. The average adult human loses 50 to 70 1,000,000,000, billion cells each day due to apoptosis. For the average human child between 8 and 14 years old, each day the approximate loss is 20 to 30 billion cells. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
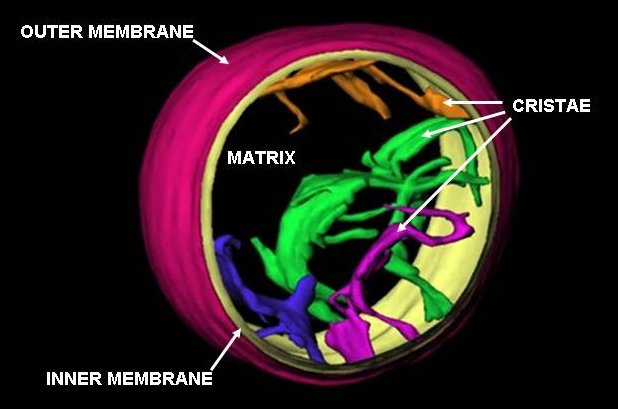
Mitochondrion
A mitochondrion () is an organelle found in the cell (biology), cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal ''Henneguya zschokkei, Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excitotoxicity
In excitotoxicity, neuron, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamic acid, glutamate become pathologically high, resulting in excessive stimulation of cell surface receptor, receptors. For example, when glutamate receptors such as the NMDA receptor or AMPA receptor encounter excessive levels of the excitatory neurotransmitter, glutamate, significant neuronal damage might ensue. Different mechanisms might lead to increased extracellular glutamate concentrations, e.g. reduced uptake by glutamate transporters (EAATs), synaptic hyperactivity, or abnormal release from different neural cell types. Excess glutamate allows high levels of calcium in biology, calcium ions (Ca2+) to enter the cell (biology), cell. Ca2+ influx into cells activates a number of enzymes, including phospholipases, endonucleases, and proteases such as calpain. These enzymes go on to damage cell structures such as components of the cytoskel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipase
A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze: *Phospholipase A ** Phospholipase A1 – cleaves the ''sn''-1 acyl chain (where ''sn'' refers to stereospecific numbering). ** Phospholipase A2 – cleaves the ''sn''-2 acyl chain, releasing arachidonic acid. * Phospholipase B – cleaves both ''sn''-1 and ''sn''-2 acyl chains; this enzyme is also known as a lysophospholipase. * Phospholipase C – cleaves before the phosphate, releasing diacylglycerol and a phosphate-containing head group. PLCs play a central role in signal transduction, releasing the second messenger inositol triphosphate. * Phospholipase D – cleaves after the phosphate, releasing phosphatidic acid and an alcohol. Types C and D are considered phosphodiesterases. Endothelial lipase is primarily a phospholipase. Phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potassium pump (Na+/K+ATPase) that maintains the cell membrane potential. Another example is the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while many, typically called '' restriction endonucleases'' or ''restriction enzymes'', cleave only at very specific nucleotide sequences. Endonucleases differ from exonucleases, which cleave the ends of recognition sequences instead of the middle (''endo'') portion. Some enzymes known as "exo-endonucleases", however, are not limited to either nuclease function, displaying qualities that are both endo- and exo-like. Evidence suggests that endonuclease activity experiences a lag compared to exonuclease activity. Restriction enzymes are endonucleases from eubacteria and archaea that recognize a specific DNA sequence. The nucleotide sequence recognized for cleavage by a restriction enzyme is called the ''restriction site''. Typically, a restriction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calpain
A calpain (; , ) is a protein belonging to the family of calcium-dependent, non-lysosomal cysteine proteases ( proteolytic enzymes) expressed ubiquitously in mammals and many other organisms. Calpains constitute the C2 family of protease clan CA in the MEROPS database. The calpain proteolytic system includes the calpain proteases, the small regulatory subunit CAPNS1, also known as CAPN4, and the endogenous calpain-specific inhibitor, calpastatin. Discovery The history of calpain's discovery originates in 1964, when calcium-dependent proteolytic activities caused by a "calcium-activated neutral protease" (CANP) were detected in brain, lens of the eye and other tissues. In the late 1960s the enzymes were isolated and characterised independently in both rat brain and skeletal muscle. These activities were caused by an intracellular cysteine protease not associated with the lysosome and having an optimum activity at neutral pH, which clearly distinguished it from the cathe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |