|
Inorganic Nonaqueous Solvent
An inorganic nonaqueous solvent is a solvent other than water, that is not an organic compound. These solvents are used in chemical research and industry for reactions that cannot occur in aqueous solutions or require a special environment. Inorganic nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia, hydrogen fluoride, sulfuric acid, as well as more specialized solvents, hydrazine, and selenium oxychloride. Protic inorganic nonaqueous solvents Prominent members include ammonia, hydrogen fluoride, sulfuric acid, hydrogen cyanide. Ammonia (and several amines as well) are useful for the generating solutions of highly reducing species because the N-H bond resists reduction. The chemistry of electrides and alkalides relies on amine solvents. The combination of HF and SbF5 is the basis of a superacid solution. Using this mixture, the conjugate acid of hydrogen sulfide ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuryl Chloride Fluoride
Sulfuryl chloride fluoride is a chemical compound with the formula . It is a colorless, easily condensed gas. It is a tetrahedral molecule. Liquified sulfuryl chloride fluoride is employed as a solvent for highly oxidizing compounds. Preparation The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite: : This salt is then chlorinated to give sulfuryl chloride fluoride : Further heating (180 °C) of potassium fluorosulfite with the sulfuryl chloride fluoride gives sulfuryl fluoride. : Alternatively, sulfuryl chloride fluoride can be prepared without using gases as starting materials by treating sulfuryl chloride with ammonium fluoride or potassium fluoride in trifluoroacetic acid Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not .... : References { ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosonium
The nitrosonium ion is , in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: , ( nitrosylsulfuric acid, more descriptively written ) and . The and salts are slightly soluble in acetonitrile . NOBF4 can be purified by sublimation at 200–250 °C and . Synthesis and spectroscopy is isoelectronic with CO, and . It arises via protonation of nitrous acid: :HONO + H+ NO+ + H2O In its infrared spectrum of its salts, νNO is a strong peak in the range 2150-2400 cm−1. Chemical properties Hydrolysis reacts readily with water to form nitrous acid: : For this reason, nitrosonium compounds must be protected from water or even moist air. With base, the reaction generates nitrite: : As a diazotizing agent reacts with aryl amines, , to give diazonium salts, . The resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autoionization
Autoionization is a process by which an atom or a molecule in an excited state spontaneously emits one of the outer-shell electrons, thus going from a state with charge to a state with charge , for example from an electrically neutral state to a singly ionized state. Autoionizing states are usually short- lived, and thus can be described as Fano resonances rather than normal bound states. They can be observed as variations in the ionization cross sections of atoms and molecules, by photoionization, electron ionization and other methods. Examples As examples, several Fano resonances in the extreme ultraviolet photoionization spectrum of neon are attributed to autoionizing states.Codling, K., Madden, R.P. and Ederer, D.L. (1967), ''Resonances in the Photoionization Continuum of Ne I (20-150 eV)'', Phys. Rev. ''155'', 26-37 DOI: https://doi.org/10.1103/PhysRev.155.26 Some are due to one-electron excitations, such as a series of three strong similarly shaped peaks at energie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arenium Ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution. For historic reasons this complex is also called a Wheland intermediate, after American chemist George Willard Wheland (1907–1976). They are also called sigma complexes. The smallest arenium ion is the benzenium ion (), which is protonated benzene. : Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms by the pi system, as depicted on the following resonance structures: : A complexed electrophile can contribute to the stability of arenium ions. Salts of benzenium ion can be isolated when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6). The benzenium salt is crystalline with thermal stability up to 150 °C. Bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocations
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However, others have more narrowly defined the term 'carbonium ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuryl Chloride Fluoride
Sulfuryl chloride fluoride is a chemical compound with the formula . It is a colorless, easily condensed gas. It is a tetrahedral molecule. Liquified sulfuryl chloride fluoride is employed as a solvent for highly oxidizing compounds. Preparation The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite: : This salt is then chlorinated to give sulfuryl chloride fluoride : Further heating (180 °C) of potassium fluorosulfite with the sulfuryl chloride fluoride gives sulfuryl fluoride. : Alternatively, sulfuryl chloride fluoride can be prepared without using gases as starting materials by treating sulfuryl chloride with ammonium fluoride or potassium fluoride in trifluoroacetic acid Trifluoroacetic acid (TFA) is a synthetic organofluorine compound with the chemical formula CF3CO2H. It belongs to the subclass of per- and polyfluoroalkyl substances (PFASs) known as ultrashort-chain perfluoroalkyl acids (PFAAs). TFA is not .... : References { ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon Difluoride
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive x ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subsequent analysis. It has also been tested as an oxidizer in liquid rocket propellants and is used as a fluorinating agent in the processing of uranium. Preparation BrF5 was first prepared in 1931 by the direct reaction of bromine and fluorine. This reaction is suitable for the preparation of large quantities, and is carried out at temperatures over with an excess of fluorine: :Br2 + 5 F2 → 2 BrF5 For the preparation of smaller amounts, potassium bromide is used: :KBr + 3 F2 → KF + BrF5 This route yields BrF5 almost completely free of trifluorides and other impurities. Reactions BrF5 reacts with water to form bromic acid and hydrofluoric acid: :BrF5 + 3 H2O → HBrO3 + 5 HF It is an extremely effective fluorinating agent, bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
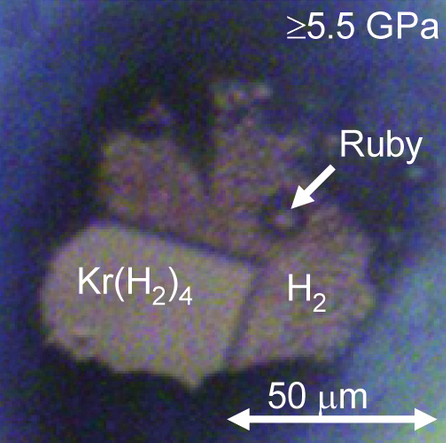
Noble Gas Compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 8 or 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton ( ionisation energy 14.0 eV), xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of or lower, in supersonic jets of noble gas, or under extremely high pressures with metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Line
The Schlenk line (also vacuum gas manifold) is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line. Schlenk lines are useful for manipulating moisture- and air-sensitive compounds. The vacuum is used to remove air or other gasses present in closed, connected glassware to the line. It often also removes the last traces of solvent from a sample. Vacuum and gas manifolds often have many ports and lines, and with care, it is possible for several reactions or operations to be run ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |