|
Heat Treating
Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term ''heat treatment'' applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding. Physical processes Photomicrographs of steel. Top: In annealed (slowly cooled) steel, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel Wikt:longulite , longulites. Structure The orientation of crystallites can be random with no preferred direction, called random Texture (chemistry), texture, or directed, possibly due to growth and processing conditions. While the structure of a single crystal is highly ordered and its crystal lattice, lattice is continuous and unbroken, amorphous solid, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a wikt:saturated#Chemistry, saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscibility, miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvation
Solvations describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration. Solubility of solid compounds dep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena. Overview Phase transitions (phase changes) that help describe polymorphism include polymorphic transitions as well as melting and vaporization transitions. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." Additionally, Walter McCrone described the phases in polymorphic matter as "different in crystal structure but identical in the liquid or vapor states." McCrone also def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical State of matter, state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are Chemical bond, bonded together in different manners. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a Cubic crystal system, cubic lattice of Tetrahedral molecular geometry, tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for Chemical compound, compounds. The more general term, used for any compound, is Polymorphism (materials science), polymorphism, although its use is usually restricted to solid materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deformation (engineering)
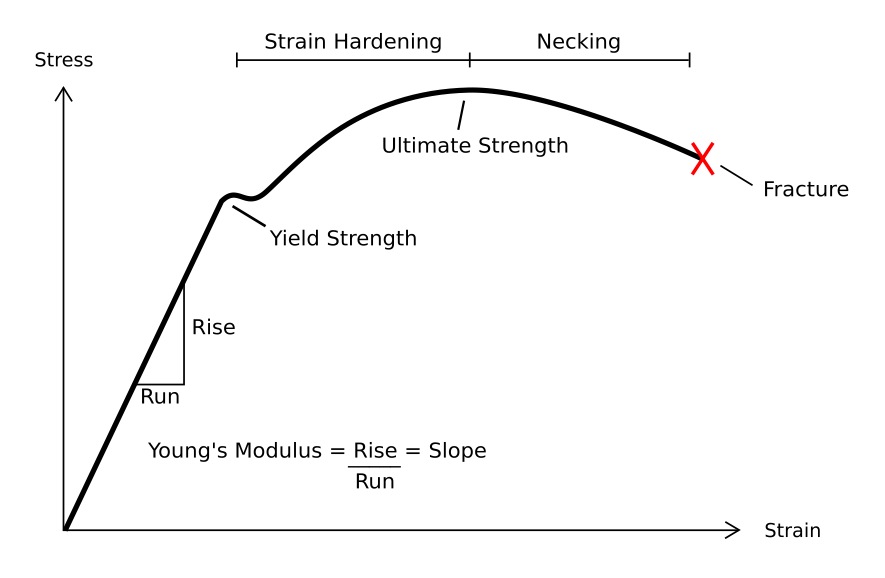
In engineering, deformation (the change in size or shape of an object) may be ''elastic'' or ''plastic''. If the deformation is negligible, the object is said to be ''rigid''. Main concepts Occurrence of deformation in engineering applications is based on the following background concepts: * ''Displacements'' are any change in position of a point on the object, including whole-body translations and rotations ( rigid transformations). * ''Deformation'' are changes in the relative position between internals points on the object, excluding rigid transformations, causing the body to change shape or size. * ''Strain'' is the ''relative'' ''internal'' deformation, the dimensionless change in shape of an infinitesimal cube of material relative to a reference configuration. Mechanical strains are caused by mechanical stress, ''see stress-strain curve''. The relationship between stress and strain is generally linear and reversible up until the yield point and the deformation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation. Properties Martensite is formed in carbon steels by the rapid cooling ( quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements. As a result of the quenching, the face-centered cubic austenite transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. The shear deformations that result produce a large number of dislocations, which is a primary strengthening mechanism of steels. The highest hardness of a pearlitic steel is 400 Brinell, whereas martensite can achieve 700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elasticity (physics)
In physics and materials science, elasticity is the ability of a body to resist a distorting influence and to return to its original size and shape when that influence or force is removed. Solid objects will deform when adequate loads are applied to them; if the material is elastic, the object will return to its initial shape and size after removal. This is in contrast to ''plasticity'', in which the object fails to do so and instead remains in its deformed state. The physical reasons for elastic behavior can be quite different for different materials. In metals, the Crystal structure, atomic lattice changes size and shape when forces are applied (energy is added to the system). When forces are removed, the lattice goes back to the original lower energy state. For rubber elasticity, rubbers and other polymers, elasticity is caused by the stretching of polymer chains when forces are applied. Hooke's law states that the force required to deform elastic objects should be Prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversible upon removing the stress. Ductility is a critical mechanical performance indicator, particularly in applications that require materials to bend, stretch, or deform in other ways without breaking. The extent of ductility can be quantitatively assessed using the percent elongation at break, given by the equation: \% \mathrm= \left ( \frac \right )\times100 where l_ is the length of the material after fracture and l_0 is the original length before testing. This formula helps in quantifying how much a material can stretch under tensile stress before failure, providing key insights into its ductile behavior. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing."Toughness" Brian Larson, editor, 2001–2011, The Collaboration for NDT Education, Iowa State University [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strength Of Materials
Strength may refer to: Personal trait *Physical strength, as in people or animals *Character strengths like those listed in the Values in Action Inventory *The exercise of willpower Physics * Mechanical strength, the ability to withstand an applied stress or load without structural failure ** Compressive strength, the capacity to withstand axially directed pushing forces **Tensile strength, the maximum stress while being stretched or pulled before necking ** Shear strength, the ability to withstand shearing * Strength (explosive), the ability of an explosive to move surrounding material * Field strength, the magnitude of a field's vector * Signal strength, in telecommunications *Strength (material), the behavior of solid objects subject to stresses and strains Music * Strength (American band), a band from Portland, Oregon * Strength (Japanese band), a band from Sendai, Miyagi, Japan * ''Strength'' (The Alarm album), 1985 * ''Strength'' (Enuff Z'nuff album), 1991 *''Stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |