|
Heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, atomic, or molecular particles, or small surface irregularities, as distinct from the macroscopic modes of energy transfer, which are thermodynamic work and transfer of matter. For a closed system (transfer of matter excluded), the heat involved in a process is the difference in internal energy between the final and initial states of a system, after subtracting the work done in the process. For a closed system, this is the formulation of the first law of thermodynamics. Calorimetry is measurement of quantity of energy transferred as heat by its effect on the states of interacting bodies, for example, by the amount of ice melted or by change in temperature of a body. In the International System of Units (SI), the unit of measurement for he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. For a thermodynamic process affecting a thermodynamic system without transfer of matter, the law distinguishes two principal forms of energy transfer, heat and thermodynamic work. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat transfer, thermodynamic work, and matter transfer, into and out of the system. Energy cannot be created or destroyed, but it can be transformed from one form to another. In an externally isolated system, with internal changes, the sum of all forms of energy is constant. An equivalent statement is that perpetual motion machines of the first kind are impossible; work done by a system on its surroundings requires that the system's internal energy be consumed, so that the amount of internal energy lost by that work must be resupplied as heat by an external e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
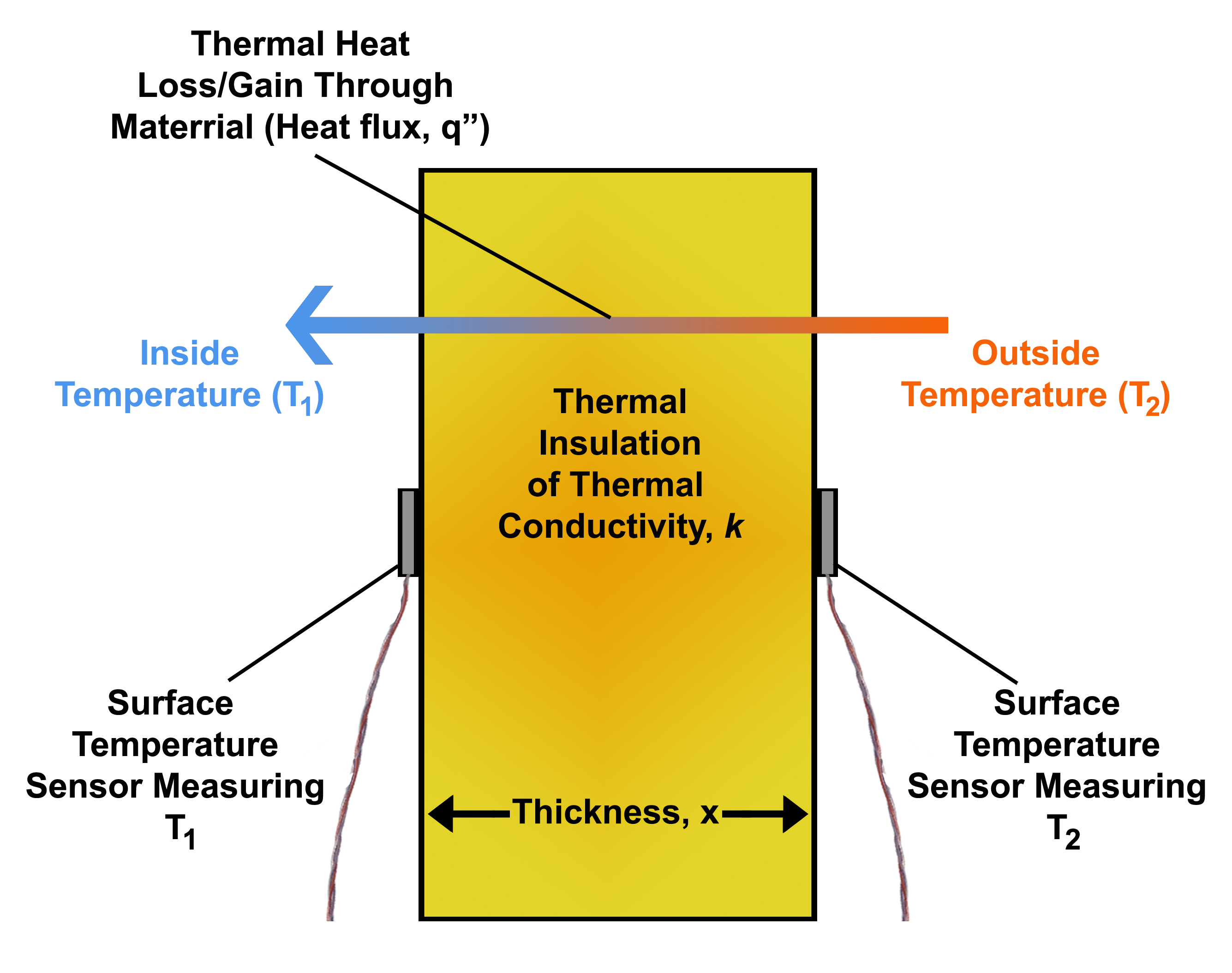
Thermal Conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy until an object has the same kinetic energy throughout. Thermal conductivity, frequently represented by , is a property that relates the rate of heat loss per unit area of a material to its rate of change of temperature. Essentially, it is a value that accounts for any property of the material that could change the way it conducts heat. Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incandescence
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electronic, molecular, and lattice oscillations in a material. Kinetic energy is converted to electromagnetism due to Larmor formula, charge-acceleration or dipole oscillation. At room temperature, most of the emission is in the infrared (IR) spectrum, though above around 525 °C (977 °F) enough of it becomes Visible spectrum, visible for the matter to visibly glow. This visible glow is called incandescence. Thermal radiation is one of the fundamental mechanisms of heat transfer, along with Thermal conduction, conduction and Convection (heat transfer), convection. The primary method by which the Sun transfers heat to the Earth is thermal radiation. This energy is partially absorbed and scattered in the atmosphere, the latter process bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in '' state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry. Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and light. Energy is a Conservation law, conserved quantity—the law of conservation of energy states that energy can be Energy transformation, converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J). Forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a Classical field theory, field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, the internal energy contained within a thermodynamic system, and rest energy associated with an object's rest mass. These are not mutual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
Thermodynamic work is one of the principal kinds of process by which a thermodynamic system can interact with and transfer energy to its surroundings. This results in externally measurable macroscopic forces on the system's surroundings, which can cause mechanical work, to lift a weight, for example,Kittel, C. Kroemer, H. (1980). ''Thermal Physics'', second edition, W.H. Freeman, San Francisco, or cause changes in electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . or gravitationalNorth, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . variables. Also, the surroundings can perform t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Flux
In physics and engineering, heat flux or thermal flux, sometimes also referred to as heat flux density, heat-flow density or heat-flow rate intensity, is a flow of energy per unit area per unit time (physics), time. Its SI units are watts per square metre (W/m2). It has both a direction and a magnitude, and so it is a Vector (geometric), vector quantity. To define the heat flux at a certain point in space, one takes the Limiting case (mathematics), limiting case where the size of the surface becomes infinitesimally small. Heat flux is often denoted \vec_\mathrm, the subscript specifying ''heat'' flux, as opposed to ''Mass flux, mass'' or Transport phenomena, ''momentum'' flux. Heat conduction#Fourier's law, Fourier's law is an important application of these concepts. Fourier's law For most solids in usual conditions, heat is transported mainly by thermal conduction, conduction and the heat flux is adequately described by Fourier's law. Fourier's law in one dimension \phi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolas Léonard Sadi Carnot
Nicolas Léonard Sadi Carnot (; 1 June 1796 – 24 August 1832) was a French people, French military engineering, military engineer and physicist. A graduate of the École polytechnique, Carnot served as an officer in the Engineering Arm (''le génie'') of the French Army. He also pursued scientific studies and in June 1824 published an essay titled ''Reflections on the Motive Power of Fire''. In that book, which would be his only publication, Carnot developed the first successful theory of the Thermal efficiency, maximum efficiency of heat engines. Carnot's scientific work attracted little attention during his lifetime, but in 1834 it became the object of a detailed commentary and explanation by another French engineer, Émile Clapeyron. Clapeyron's commentary in turn attracted the attention of William Thomson, 1st Baron Kelvin, William Thomson (later Lord Kelvin) and Rudolf Clausius. Thomson used Carnot's analysis to develop an absolute thermodynamic temperature scale, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accounting for the gains and losses of energy due to changes in its internal state, including such quantities as magnetization. It excludes the kinetic energy of motion of the system as a whole and the potential energy of position of the system as a whole, with respect to its surroundings and external force fields. It includes the thermal energy, ''i.e.'', the constituent particles' kinetic energies of motion relative to the motion of the system as a whole. Without a thermodynamic process, the internal energy of an isolated system cannot change, as expressed in the law of conservation of energy, a foundation of the first law of thermodynamics. The notion has been introduced to describe the systems characterized by temperature variations, te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Thermal Unit
The British thermal unit (Btu) is a measure of heat, which is a form of energy. It was originally defined as the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. It is also part of the United States customary units. The SI unit for energy is the joule (J); one Btu equals about 1,055 J (varying within the range of 1,054–1,060 J depending on the specific definition of Btu; see below). While units of heat are often supplanted by energy units in scientific work, they are still used in some fields. For example, in the United States the price of natural gas is quoted in dollars per the amount of natural gas that would give 1 million Btu (1 "MMBtu") of heat energy if burned. Definitions A Btu was originally defined as the amount of heat required to raise the temperature of one pound of liquid water by one degree Fahrenheit at a constant pressure of one atmospheric unit. There are several different definitions of the Btu tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |