|
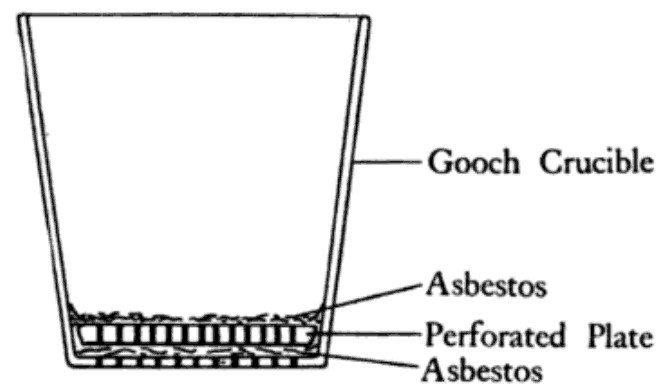
Gooch Crucible
{{Short description, Filtration device A Gooch crucible, named after Frank Austin Gooch,Classic Kit: Gooch's Cruciblby Dr. Andrea Sella is a filtration device for laboratory use (and was also called a Gooch filterJ. Am. Chem. Soc., 1882, 4 (8), p 161). It is convenient for collecting a precipitate directly within the vessel in which it is to be dried, possibly Ash (analytical chemistry), ashed, and finally weighed in gravimetric analysis. The device was originally a standard platinum laboratory crucible with a perforated base into which asbestos pulp was placed to form the filter mat. The crucible was then heated in an oven to dry out until it attained constant weight. The use of these materials meant that after filtration, the crucible and its contents could be subjected to high temperature to dry the filtrate and possibly oxidize or ash it to minimum weight. However, because of the high cost of platinum, versions made of porcelain were introduced in 1882. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frank Austin Gooch
Frank Austin Gooch (1852 – 1929) was an American chemist and engineer. Biography He was born to Joshua G. and Sarah Gates (Coolidge) Gooch in Watertown, Massachusetts. On his mother's side of the family, he was a descendant of Thomas Hastings (colonist), Thomas Hastings who came from the East Anglia region of England to the Massachusetts Bay Colony in 1634. Gooch invented the Gooch crucible, which is used, for example, to determine the solubility of bituminous materials such as road tars and petroleum asphalts. He was awarded a Ph.D. by Harvard University in 1877. Gooch was a professor of chemistry at Yale University from 1885 to 1918. He devised or perfected a large number of analytical processes and methods, including: * Invented the Gooch crucible, Gooch filtering crucible. * Studied the quantitative separation of lithium from the other alkali metals, and the estimation of boric acid by distillation with methanol and fixation by calcium oxide. * Developed methods f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ash (analytical Chemistry)
In analytical chemistry, ashing or ash content determination is the process of Mineralization (geology), mineralization by complete combustion for preconcentration of trace substances prior to a chemical analysis, such as chromatography, or optical analysis, such as spectroscopy. Overview The ash content of a sample is a measure of the amount of inorganic noncombustible material it contains. The residues after a sample is completely combustion, burnt - in contrast to the ash remaining after incomplete combustion - typically consist of oxides of the inorganic elements present in the original sample. Ash is one of the components in the proximate analysis of biological materials, consisting mainly of salty, inorganic constituents. It includes metal salt (chemistry), salts which are important for processes requiring ions such as Na+ (sodium), K+ (potassium), and Ca2+ (calcium). It also includes trace minerals which are required for unique molecules, such as chlorophyll and hemoglobin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravimetric Analysis
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known. The four main types of this method of analysis are ''precipitation'', ''volatilization'', ''electro-analytical'' and ''miscellaneous physical method''. The methods involve changing the phase of the analyte to separate it in its pure form from the original mixture and are quantitative measurements. Precipitation method The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H2C2O4, is added to a measured, known volume of water. By adding a reagent, here ammon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name originates from Spanish language, Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 element, group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the Abundance of elements in Earth's crust, rarer elements in Earth's crust, with an average abundance of approximately 5 microgram, μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some Native element mineral, native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury Basin, Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asbestos
Asbestos ( ) is a group of naturally occurring, Toxicity, toxic, carcinogenic and fibrous silicate minerals. There are six types, all of which are composed of long and thin fibrous Crystal habit, crystals, each fibre (particulate with length substantially greater than width) being composed of many microscopic "fibrils" that can be released into the atmosphere by Abrasion (mechanical), abrasion and other processes. Inhalation of asbestos fibres can lead to various dangerous lung conditions, including mesothelioma, asbestosis, and lung cancer. As a result of these health effects, asbestos is considered a serious Health hazard, health and safety hazard. Archaeological studies have found evidence of asbestos being used as far back as the Stone Age to strengthen ceramic pots, but large-scale mining began at the end of the 19th century when manufacturers and builders began using asbestos for its desirable physical properties. Asbestos is an excellent Thermal insulation, thermal and In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porcelain
Porcelain (), also called china, is a ceramic material made by heating Industrial mineral, raw materials, generally including kaolinite, in a kiln to temperatures between . The greater strength and translucence of porcelain, relative to other types of pottery, arise mainly from Vitrification#Ceramics, vitrification and the formation of the mineral mullite within the body at these high temperatures. End applications include tableware, ceramic art, decorative ware such as figurines, and products in technology and industry such as Insulator (electricity), electrical insulators and laboratory ware. The manufacturing process used for porcelain is similar to that used for earthenware and stoneware, the two other main types of pottery, although it can be more challenging to produce. It has usually been regarded as the most prestigious type of pottery due to its delicacy, strength, and high degree of whiteness. It is frequently both glazed and decorated. Though definitions vary, po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspirator (pump)
A vacuum ejector, or simply ejector, or aspirator, is a type of vacuum pump, which produces vacuum by means of the Venturi effect. In an ejector, a working fluid (liquid or gaseous) flows through a jet nozzle into a tube that first narrows and then expands in cross-sectional area. The fluid leaving the jet is flowing at a high velocity which due to Bernoulli's principle results in it having low pressure, thus generating a vacuum. The outer tube then narrows into a mixing section where the high velocity working fluid mixes with the fluid that is drawn in by the vacuum, imparting enough velocity for it to be ejected, the tube then typically expands in order to decrease the velocity of the ejected stream, allowing the pressure to smoothly increase to the external pressure. The strength of the vacuum produced depends on the velocity and shape of the fluid jet and the shape of the constriction and mixing sections, but if a liquid is used as the working fluid, the strength of the va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Büchner Flask
A Büchner flask, also known as a vacuum flask,The use of the term ''vacuum flask'' sometimes causes confusion with the thermos flask filter flask, suction flask, side-arm flask, or Bunsen flask, is a thick-walled Erlenmeyer flask with a short glass tube and hose barb protruding about 1-2 cm from its neck. Description The short tube and hose barb effectively act as an adapter over which the end of a thick-walled hose can be fitted to form a connection to the flask. The other end of the hose can be connected to source of vacuum such as an aspirator, vacuum pump, or house vacuum. Preferably this is done through a trap (see below), which is designed to prevent the water to be sucked back from the aspirator into the Büchner flask. The purpose of applying a vacuum is to speed the filtration by providing a pressure differential across the filter medium that is greater than that produced by gravity alone. Operation The thick wall of the Büchner flask provides it the strength to withs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |