|
Geochemistry Of Carbon
The geochemistry of carbon is the study of the transformations involving the element carbon within the systems of the Earth. To a large extent this study is organic geochemistry, but it also includes the very important carbon dioxide. Carbon is transformed by life, and moves between the major phases of the Earth, including the water bodies, atmosphere, and the rocky parts. Carbon is important in the formation of organic mineral deposits, such as coal, petroleum or natural gas. Most carbon is cycled through the atmosphere into living organisms and then respirated back into the atmosphere. However an important part of the carbon cycle involves the trapping of living matter into sediments. The carbon then becomes part of a sedimentary rock when lithification happens. Human technology or natural processes such as weathering, or underground life or water can return the carbon from sedimentary rocks to the atmosphere. From that point it can be transformed in the rock cycle into metamorphic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
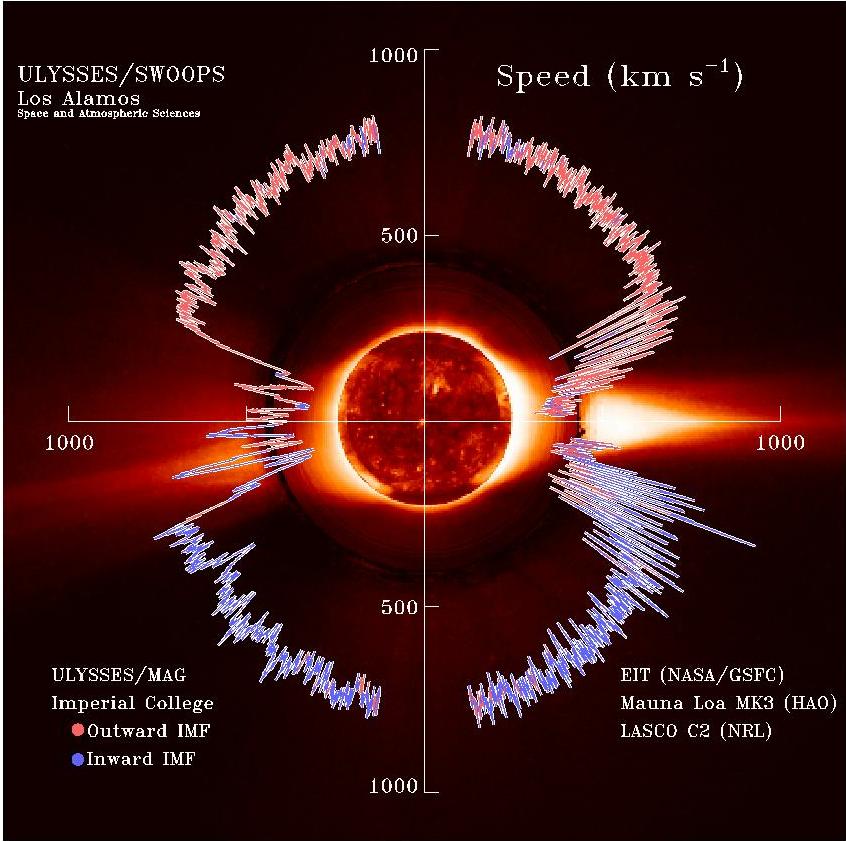
Solar Wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of particle species found in the solar plasma: trace amounts of heavy ions and atomic nuclei of Chemical element, elements such as carbon, nitrogen, oxygen, neon, magnesium, silicon, sulfur, and iron. There are also rarer traces of some other nuclei and isotopes such as phosphorus, titanium, chromium, and nickel's isotopes 58Ni, 60Ni, and 62Ni. Superimposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over Solar coordinate systems#Heliographic, solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonaceous Chondrite
Carbonaceous chondrites or C chondrites are a class of chondritic meteorites comprising at least 8 known groups and many ungrouped meteorites. They include some of the most primitive known meteorites. The C chondrites represent only a small proportion (4.6%) of meteorite falls. Some famous carbonaceous chondrites are: Allende, Murchison, Orgueil, Ivuna, Murray, Tagish Lake, Sutter's Mill, and Winchcombe. General description C chondrites contain a relatively high proportion of carbon (up to 3%), which is in the form of graphite, carbonates and organic compounds, including amino acids. In addition, they contain water and minerals that have been modified by the influence of water. The carbonaceous chondrites were not exposed to higher temperatures, so that they are hardly changed by thermal processes. Some carbonaceous chondrites, such as the Allende meteorite, contain calcium-aluminum-rich inclusions (CAIs). These are compounds that emerged early from the primeval solar n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interplanetary Dust
The interplanetary dust cloud, or zodiacal cloud (as the source of the zodiacal light), consists of cosmic dust (small particles floating in outer space) that pervades the space between planets within planetary systems, such as the Solar System. This system of particles has been studied for many years in order to understand its nature, origin, and relationship to larger bodies. There are several methods to obtain space dust measurement. In the Solar System, interplanetary dust particles have a role in scattering sunlight and in emitting thermal radiation, which is the most prominent feature of the night sky's radiation, with wavelengths ranging 5–50 μm. The particle sizes of grains characterizing the infrared emission near Earth's orbit typically range 10–100 μm. Microscopic impact craters on lunar rocks returned by the Apollo Program revealed the size distribution of cosmic dust particles bombarding the lunar surface. The ’’Grün’’ distribution of interplanetar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meteorite
A meteorite is a rock (geology), rock that originated in outer space and has fallen to the surface of a planet or Natural satellite, moon. When the original object enters the atmosphere, various factors such as friction, pressure, and chemical interactions with the atmospheric gases cause it to heat up and radiate energy. It then becomes a meteor and forms a Meteoroid#Fireball, fireball, also known as a shooting star; astronomers call the brightest examples "Bolide#Astronomy, bolides". Once it settles on the larger body's surface, the meteor becomes a meteorite. Meteorites vary greatly in size. For geologists, a bolide is a meteorite large enough to create an impact crater. Meteorites that are recovered after being observed as they transit the atmosphere and impact event, impact Earth are called meteorite falls. All others are known as meteorite finds. Meteorites have traditionally been divided into three broad categories: stony meteorites that are rocks, mainly composed of sil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstellar Medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic medium. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. Although the density of atoms in the ISM is usually far below that in the best laboratory vacuums, the mean free path between collisions is short compared to typical interstellar lengths, so on these scales the ISM behaves as a gas (more precisely, as a Plasma (physics), plasma: it is everywhere at least slightly ionized), responding to pressure forces, and not as a collection of non-interacting particles. The interstellar medium is composed of multiple phases distinguished by whether matter is ionic, atomic, or molecular, and the temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trihydrogen Cation
The trihydrogen cation or protonated molecular hydrogen ( IUPAC name: hydrogen onium ion) is a cation (positive ion) with formula , consisting of three hydrogen nuclei (protons) sharing two electrons. The trihydrogen cation is one of the most abundant ions in the universe. It is stable in the interstellar medium (ISM) due to the low temperature and low density of interstellar space. The role that plays in the gas-phase chemistry of the ISM is unparalleled by any other polyatomic ion. The trihydrogen cation is the simplest triatomic molecule, because its two electrons are the only valence electrons in the system. It is also the simplest example of a three-center two-electron bond system. History was first discovered by J. J. Thomson in 1911. While using an early form of mass spectrometry to study the resultant species of plasma discharges, he discovered a large abundance of a polyatomic ion with a mass-to-charge ratio of 3. He stated that the only two possibilities were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest of the allotropes of carbon (after atomic carbon), and is an intermediate participant in the genesis of fullerenes. Properties C2 is a component of carbon vapor. One paper estimates that carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. Electromagnetic properties The electrons in diatomic carbon are distributed among the molecular orbitals according to the Aufbau principle to produce unique quantum states, with corresponding energy levels. The state with the lowest energy le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, H2), and the formation of H II regions. This is in contrast to other areas of the interstellar medium that contain predominantly ionized gas. Molecular hydrogen is difficult to detect by infrared and radio observations, so the molecule most often used to determine the presence of H2 is carbon monoxide (CO). The ratio between CO luminosity and H2 mass is thought to be constant, although there are reasons to doubt this assumption in observations of some other galaxies. Within molecular clouds are regions with higher density, where much dust and many gas cores reside, called clumps. These clumps are the beginning of star formation if gravitational forces are sufficient to cause the dust and gas to collapse. Research and discovery The histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |