|
Field-emission Microscope
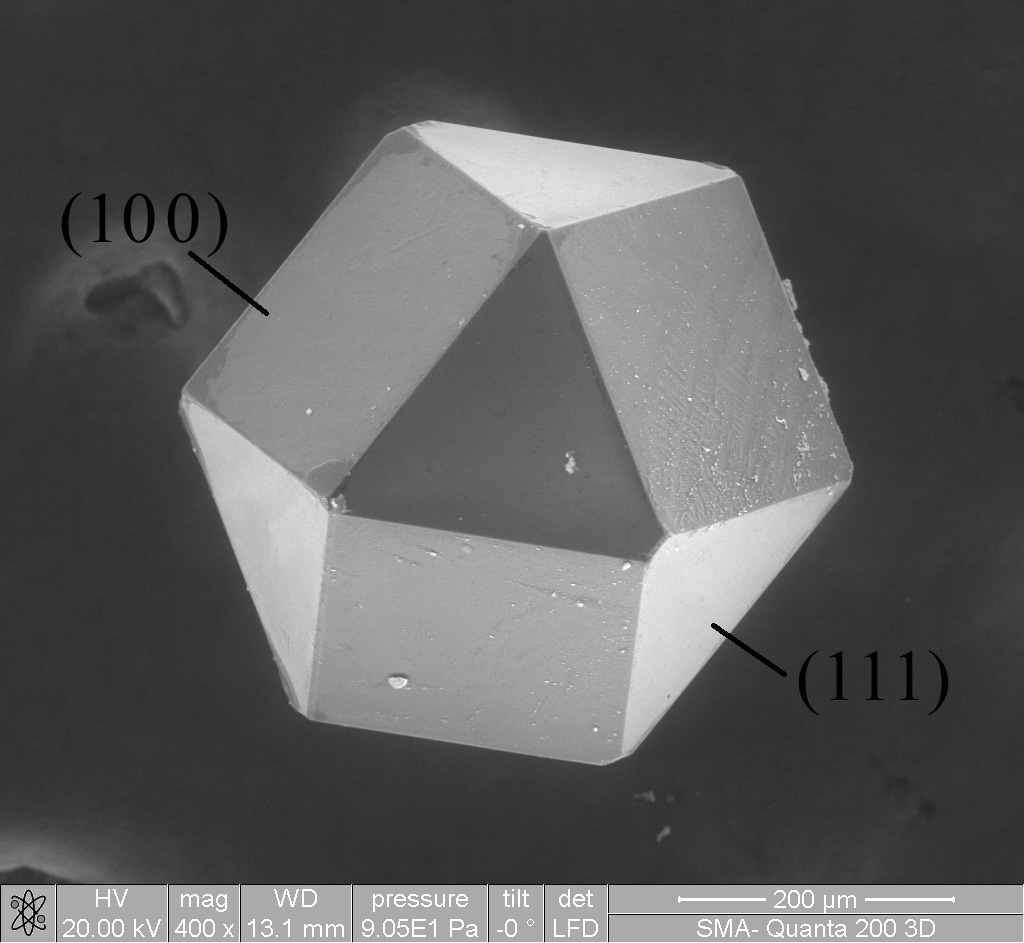
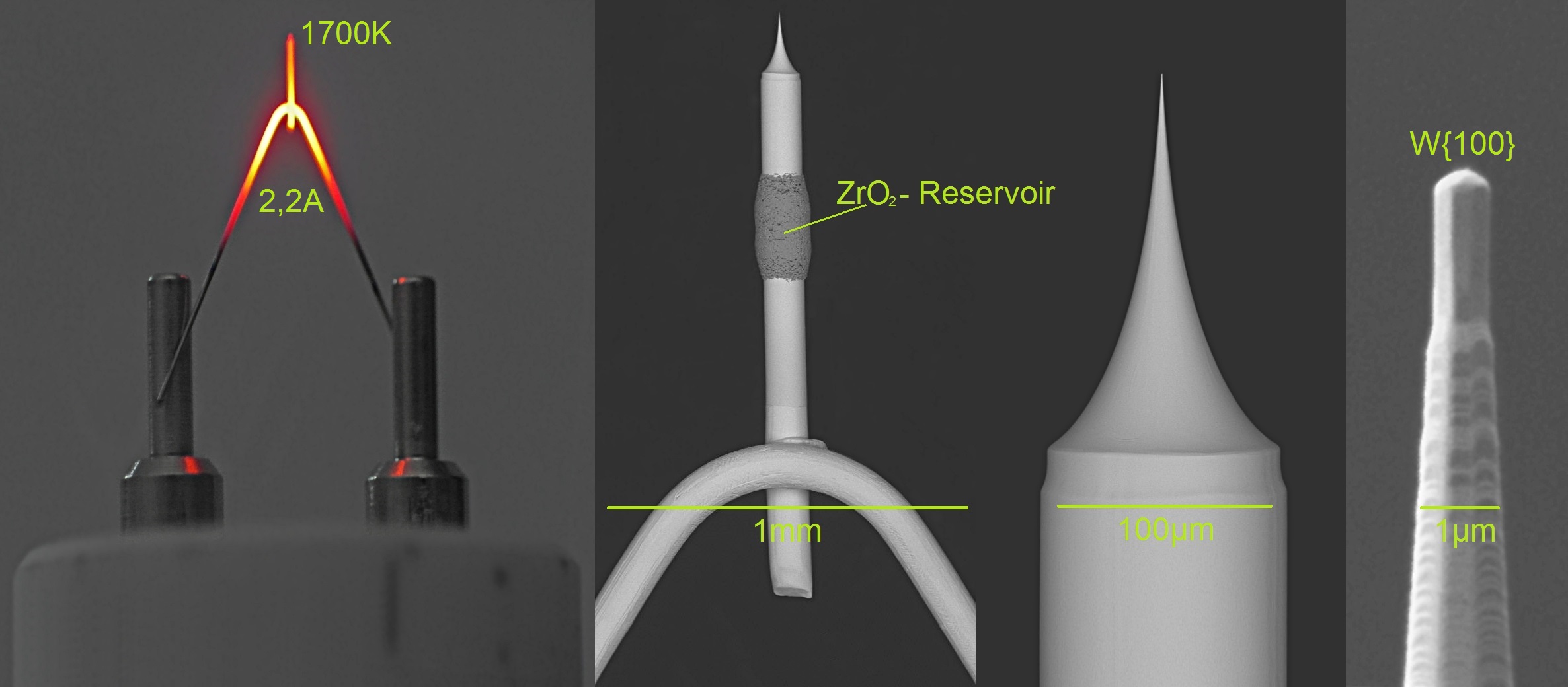
Field-emission microscopy (FEM) is an analytical technique that is used in materials science to study the surfaces of needle apexes. The FEM was invented by Erwin Wilhelm Müller in 1936, and it was one of the first surface-analysis instruments that could approach near-atomic resolution. Introduction Microscopy techniques are utilized to generate magnified real-space images of the surface of a tip apex. Typically, microscopy information pertains to the surface crystallography (i.e., how the atoms are arranged at the surface) and surface morphology (i.e., the shape and size of topographic features making the surface). Field-emission microscopy (FEM) was invented by Erwin Müller in 1936. In FEM, the phenomenon of field electron emission was used to obtain an image on the detector based on the difference in work function of the various crystallographic planes on the surface. Setup and working principle A field-emission microscope consists of a metallic sample shaped like a sharp t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Materials Science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries. The intellectual origins of materials science stem from the Age of Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Materials science still incorporates elements of physics, chemistry, and engineering. As such, the field was long considered by academic institutions as a sub-field of these related fields. Beginning in the 1940s, materials science began to be more widely recognized as a specific and distinct field of science and engineering, and major technical universities around the world created dedicated schools for its study. Materials scientists emphasize understanding how the history of a material (''processing'') influences its struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work Function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination). Definition The work function for a given surface is defined by the difference :W = -e\phi - E_, where is the charge of an electron, is the electrostatic potential in the vacuum nearby the surface, and is the Fermi level (electrochemical potential of electrons) inside the material. The term is the energy of an electron at rest in the vacuum nearby the surface. In practice, one directly controls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Microscopy Techniques
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up and down quarks. Electrons are extremely lightweight particles that orbit the positively charged nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electrons are the least tightly bound and are responsible for the formation of chemical bonds between atoms to create molecules and crystals. These valence electrons als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Surface Analysis Methods
This is a list of analysis methods used in materials science. Analysis methods are listed by their acronym, if one exists. Symbols * μSR – see muon spin spectroscopy * χ – see magnetic susceptibility A * AAS – Atomic absorption spectroscopy * AED – Auger electron diffraction * AES – Auger electron spectroscopy * AFM – Atomic force microscopy * AFS – Atomic fluorescence spectroscopy * Analytical ultracentrifugation * APFIM – Atom probe field ion microscopy * APS – Appearance potential spectroscopy * ARPES – Angle resolved photoemission spectroscopy * ARUPS – Angle resolved ultraviolet photoemission spectroscopy * ATR – Attenuated total reflectance B * BET – BET surface area measurement (BET from Brunauer, Emmett, Teller) * BiFC – Bimolecular fluorescence complementation * BKD – Backscatter Kikuchi diffraction, see EBSD * BRET – Bioluminescence resonance energy transfer * BSED – Back scattered electron diffraction, see EBSD C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Field Ion Microscope
The field-ion microscope (FIM) was invented by Müller in 1951. It is a type of microscope that can be used to image the arrangement of atoms at the surface of a sharp metal tip. On October 11, 1955, Erwin Müller and his Ph.D. student, Kanwar Bahadur (Pennsylvania State University) observed individual tungsten atoms on the surface of a sharply pointed tungsten tip by cooling it to 21 K and employing helium as the imaging gas. Müller & Bahadur were the first persons to observe individual atoms directly. Introduction In FIM, a sharp (<50 nm tip radius) metal tip is produced and placed in an ultra high vacuum chamber, which is backfilled with an imaging gas such as or |
Electron Microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it to produce magnified images or electron diffraction patterns. As the wavelength of an electron can be up to 100,000 times smaller than that of visible light, electron microscopes have a much higher Angular resolution, resolution of about 0.1 nm, which compares to about 200 nm for optical microscope, light microscopes. ''Electron microscope'' may refer to: * Transmission electron microscopy, Transmission electron microscope (TEM) where swift electrons go through a thin sample * Scanning transmission electron microscopy, Scanning transmission electron microscope (STEM) which is similar to TEM with a scanned electron probe * Scanning electron microscope (SEM) which is similar to STEM, but with thick samples * Electron microprobe sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom Probe
The atom probe was introduced at th14th Field Emission Symposium in 1967by Erwin Wilhelm Müller and J. A. Panitz. It combined a field ion microscope with a mass spectrometer having a single particle detection capability and, for the first time, an instrument could “... determine the nature of one single atom seen on a metal surface and selected from neighboring atoms at the discretion of the observer”. Atom probes are unlike conventional optical or electron microscopes, in that the magnification effect comes from the magnification provided by a highly curved electric field, rather than by the manipulation of radiation paths. The method is destructive in nature removing ions from a sample surface in order to image and identify them, generating magnifications sufficient to observe individual atoms as they are removed from the sample surface. Through coupling of this magnification method with time of flight mass spectrometry, ions evaporated by application of electric pulses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Diffusion
Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters ( adparticles) at solid material surfaces.Oura, Lifshits, Saranin, Zotov, and Katayama 2003, p. 325 The process can generally be thought of in terms of particles jumping between adjacent adsorption sites on a surface, as in figure 1. Just as in bulk diffusion, this motion is typically a thermally promoted process with rates increasing with increasing temperature. Many systems display diffusion behavior that deviates from the conventional model of nearest-neighbor jumps. Tunneling diffusion is a particularly interesting example of an unconventional mechanism wherein hydrogen has been shown to diffuse on clean metal surfaces via the quantum tunneling effect. Various analytical tools may be used to elucidate surface diffusion mechanisms and rates, the most important of which are field ion microscopy and scanning tunneling microscopy.Oura, Lifshits, Saranin, Zotov, and Katayama 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term '' sorption'' encompasses both adsorption and absorption, and ''desorption'' is the reverse of sorption. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractory Metals
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definitions of which elements belong to this group differ. The most common definition includes five elements: two of the fifth period (niobium and molybdenum) and three of the sixth period (tantalum, tungsten, and rhenium). They all share some properties, including a melting point above 2000 °C and high hardness at room temperature. They are chemically inert and have a relatively high density. Their high melting points make powder metallurgy the method of choice for fabricating components from these metals. Some of their applications include tools to work metals at high temperatures, wire filaments, casting molds, and chemical reaction vessels in corrosive environments. Partly due to their high melting points, refractory metals are stable against creep deformation to very high tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Field Electron Emission
Field electron emission, also known as field-induced electron emission, field emission (FE) and electron field emission, is the emission of electrons from a material placed in an electrostatic field. The most common context is field emission from a solid surface into a vacuum. However, field emission can take place from solid or liquid surfaces, into a vacuum, a fluid (e.g. air), or any non-conducting or weakly conducting dielectric. The field-induced promotion of electrons from the valence (chemistry), valence to conduction band of semiconductors (the Zener effect) can also be regarded as a form of field emission. Field emission in pure metals occurs in high electric fields: the gradients are typically higher than 1 gigavolt per metre and strongly dependent upon the work function. While electron sources based on field emission have a number of applications, field emission is most commonly an undesirable primary source of electrical breakdown, vacuum breakdown and electrical disch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erwin Wilhelm Müller
Erwin Wilhelm Müller (or ''Mueller'') (June 13, 1911 – May 17, 1977) was a German physicist who invented the Field Emission Electron Microscope (FEEM), the Field Ion Microscope (FIM), and the Atom-Probe Field Ion Microscope. He and his student, Kanwar Bahadur, were the first people to experimentally observe atoms. Life and work Müller was born in Berlin, where he studied at the Technische Hochschule in Charlottenburg (now Technische Universität Berlin) under Gustav Hertz. He received his degree in engineering in 1935 and his doctorate in 1936. Müller worked at the Siemens Research Laboratory, where he invented the field emission microscope in 1936 that allowed resolutions of 2 nanometers. Müller married Klara Thüssing in 1939, and their only daughter Jutta was born in 1940. Due to the circumstances of war, he also worked at the Stabilovolt Company. In 1947, he was appointed to the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry (renamed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |