|
Fecal Microbiota
Fecal microbiota, sold under the brand name, Rebyota is used for the prevention of recurrence of ''Clostridioides difficile'' infection. The most commonly reported adverse reactions include abdominal pain, diarrhea, abdominal distention, flatulence, and nausea. Fecal microbiota is prepared from stool donated by qualified individuals. The donors and the donated stool are tested for a panel of transmissible pathogens. Fecal microbiota is the first fecal microbiota product approved by the US Food and Drug Administration (FDA). Fecal microbiota was approved for medical use in the United States in November 2022. Medical uses Fecal microbiota is approved for the prevention of recurrence of ''Clostridioides difficile'' infection (CDI) in people 18 years of age and older. It is for use after an individual has completed antibiotic treatment for recurrent CDI. History The safety of fecal microbiota was assessed from two randomized, double-blind, placebo-controlled clinical studie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferring Pharmaceuticals
Ferring Pharmaceuticals is a Swiss multinational biopharmaceutical company specialising in areas such as reproductive health, maternal health, gastroenterology and urology. Ferring has been developing treatments for mothers and babies for over 50 years. Headquartered in Saint-Prex, Switzerland, Ferring has its own manufacturing facilities in several European countries, in South America, China, India, and the United States. Founded in 1950, privately-owned Ferring employs over 7,000 employees worldwide, has its own operating subsidiaries in more than 50 countries and markets its products in over 100 countries. History The company was founded by Dr. Frederik Paulsen Sr and Dr. Eva Paulsen in Malmö, Sweden, in 1950, initially as the Nordiska Hormon Laboratoriet, renamed Ferring in 1954. A ''ferring'' in Frisian is a person from the island Föhr off the western coast of Germany. Mr. Paulsen's family originates from that island. The first major breakthrough was the synthetic pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rectal Administration
Rectal administration (colloquially known as boofing or plugging) uses the rectum as a route of administration for medication and other fluids, which are absorbed by the rectum's blood vessels,The rectum has numerous blood vessels available to absorb drugs: upwards 2/3rds of the dose bypasses first-pass metabolism through systemic distribution and the rest is taken through the liver and metabolized via the hepatic portal system. and flow into the body's circulatory system, which distributes the drug to the body's organs and bodily systems.The organs and systems include, depending on if the drug is able to pass the blood–brain barrier (BBB) or not, the central nervous system (CNS), peripheral nervous system (PNS), cardiovascular system (CVS), et cetera. Uses Medical uses In addition to pharmacological effects, rectal administration has some properties which can be advantageous for the use in medicine. Rectal administration can allow patients to remain in the home setting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Canada
Health Canada (HC; )Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary units, department of the Government of Canada responsible for national health policy. The department itself is also responsible for numerous federal health-related agencies, including the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), among others. These organizations help to ensure compliance with federal law in a variety of Healthcare in Canada, healthcare, Agriculture in Canada, agricultural, and Pharmaceutics, pharmaceutical activities. This responsibility also involves extensive collaboration with various other federal- and provincial-level organizations in order to ensure the safety of food, health, and Medication, pharmaceutical products—including the regulation of health research and pharmaceutical manufacturing/Clinical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
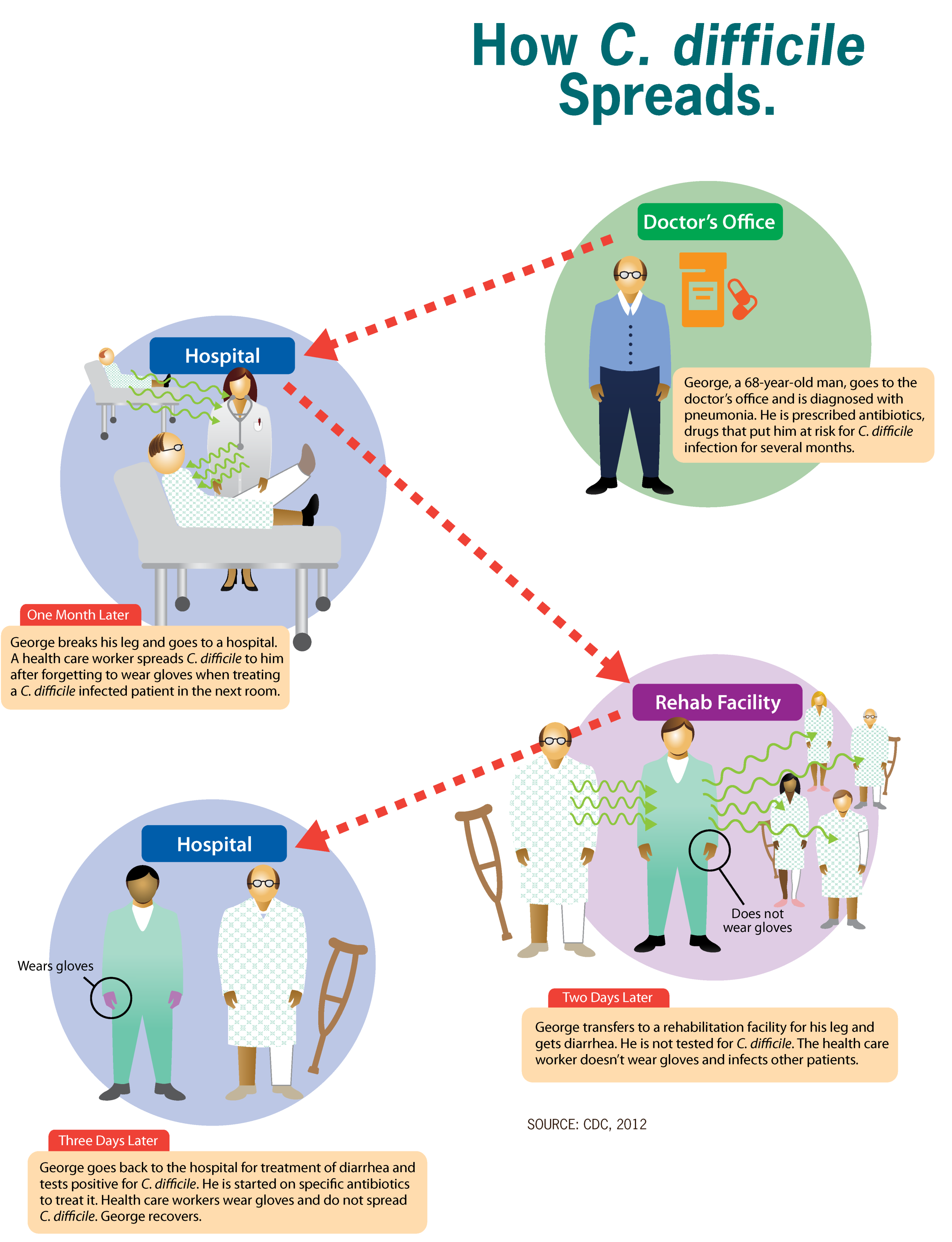
Clostridioides Difficile Infection
''Clostridioides difficile'' infection (CDI or C-diff), also known as ''Clostridium difficile'' infection, is a symptomatic infection due to the bacterial spores, spore-forming bacterium ''Clostridioides difficile''. Symptoms include watery diarrhea, fever, nausea, and abdominal pain. It makes up about 20% of cases of antibiotic-associated diarrhea. Antibiotics can contribute to detrimental changes in gut microbiota; specifically, they decrease short-chain fatty acid absorption, which results in osmotic, or watery, diarrhea. Complications may include colitis#Infectious, pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis. ''Clostridioides difficile'' infection is spread by bacterial spores found within feces. Surfaces may become contaminated with the spores, with further spread occurring via the hands of healthcare workers. Risk factors for infection include antibiotic or proton pump inhibitor use, hospitalization, hypoalbuminemia, other health pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast track is one of five FDA approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and regenerative medicine advanced therapy. Fast track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. Serious condition: det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |