|
Evans–Tishchenko Reaction
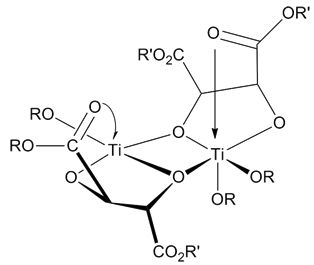
The Evans–Tishchenko reaction is the diastereoselective reduction of β-hydroxy ketones to the corresponding 1,3-''anti'' diol monoesters. The reaction employs a Lewis acid, often samarium iodide, and an aldehyde. It was first described in 1990 by David A. Evans and Amir Hoveyda, as a development of the well-known Tishchenko reaction discovered in 1906. The Aldol–Tishchenko reaction provides another potential route to 1,3-diol monoester products. Mechanism The reaction mechanism involves the attack of the aldehyde from the free alcohol group. The Lewis acid can then chelate between the two oxygen atoms to form a cyclic, 6-membered transition state. The hydride, formerly the formyl hydrogen on the aldehyde, is delivered intramolecularly, accounting for the observed ''anti'' diastereoselectivity: the result is a 1,3-''anti'' diol monoester. The proposed mechanism is further supported by isotopic labeling, which demonstrates that the formyl hydrogen is the one that migrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereoselective
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evans–Saksena Reduction
The Saksena–Evans reduction is a diastereoselective reduction of β-hydroxy ketones to the corresponding ''anti''-dialcohols, employing the reagent tetramethylammonium triacetoxyborohydride (Me4NHB(OAc)3). The reaction was first described by Anil K. Saksena in 1983 and further developed by David A. Evans in 1987. The reaction is thought to proceed through the 6-membered ring transition state shown below. The intramolecular hydride delivery from the boron reducing agent forces the reduction to proceed from the opposite face of the chelating β-alcohol, thus determining the diastereoselectivity. This can be contrasted with the Narasaka–Prasad reduction which similarly employs a boron chelating agent but undergoes an intermolecular hydride delivery, favouring the corresponding ''syn''-diol product. The Saksena-Evans reduction has since been used in the synthesis of several products, particularly the bryostatin Bryostatins are a group of macrolide lactones from the marine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Narasaka–Prasad Reduction
The Narasaka–Prasad reduction, sometimes simply called Narasaka reduction, is a diastereoselective reduction of β-hydroxy ketones to the corresponding ''syn''- dialcohols. The reaction employs a boron chelating agent, such as BBu2OMe, and a reducing agent, commonly sodium borohydride. This protocol was first discovered by Narasaka in 1984. The reaction proceeds through the 6-membered transition state shown below. Chelation by the boron agent favors hydride delivery from the top face because it leads directly to the more stable chair-like conformation of the product ( Fürst-Plattner Rule). The intermolecular hydride delivery from NaBH4 therefore proceeds via an axial attack from the opposite face with respect to the existing alcohol. This reaction can be contrasted with the similar Evans–Saksena reduction that employs a different boron reagent in order to achieve intramolecular hydride delivery from the same face of the alcohol, thus producing the ''anti''-diol. The Nara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis (journal)
''Synthesis'' is a scientific journal published from 1969 to the present day by Thieme. Its stated purpose is the "advancement of the science of synthetic chemistry". From August 2006, selected articles are offered free of charge. The impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of this journal is 2.867 (2018).Journal Citation Reports, 2018 References Chemistry journals English-language journals Thieme academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling. In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state. Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |