|
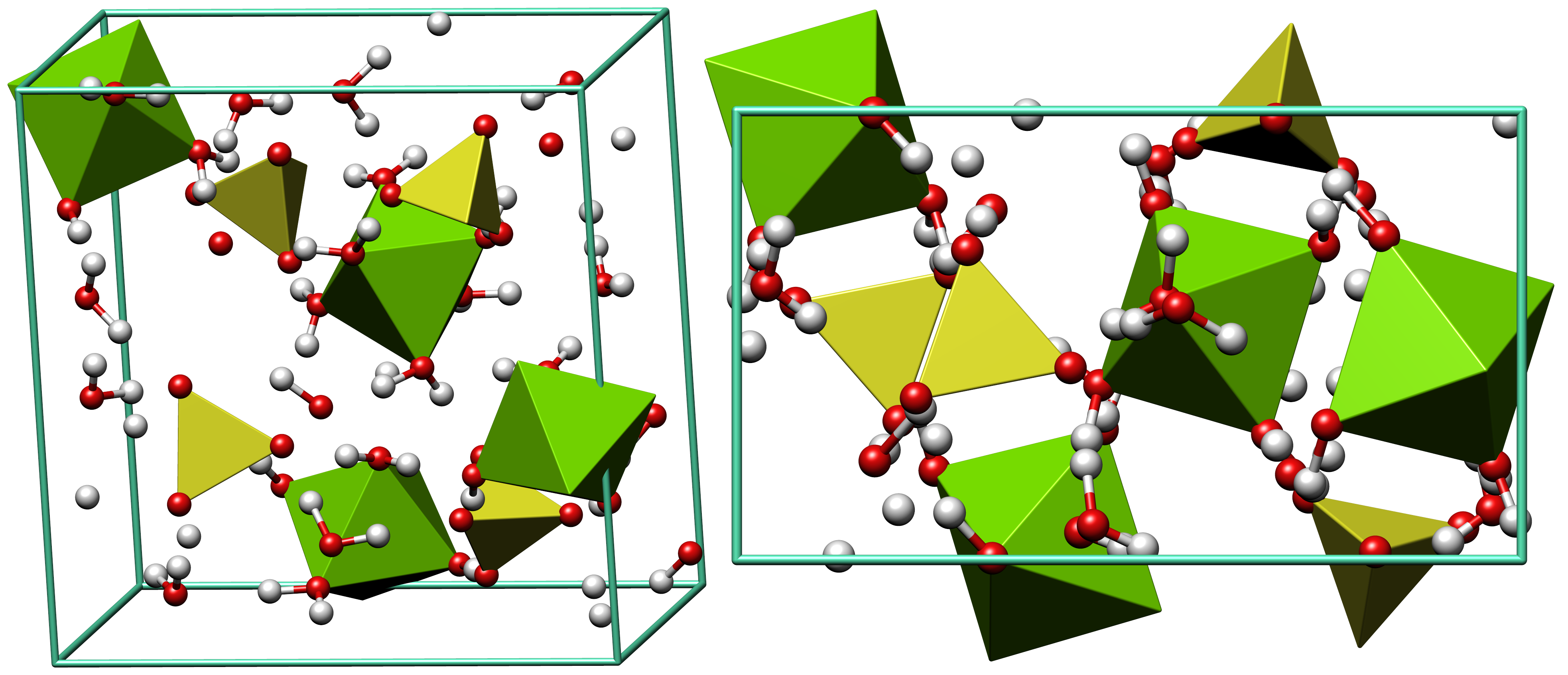
Epsom Salt
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula . Physical properties Epsomite crystallizes in the orthorhombic system. The normal form is as massive encrustations, while acicular or fibrous crystals are rarely found. It is colorless to white with tints of yellow, green and pink. It is a soft mineral with variable Mohs hardness around 2.0~2.5, and it has a low specific gravity It is readily soluble in water, and absorbs water from the air. It converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. The epsomite group includes solid solution series with morenosite (·) and goslarite (·). Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. It has been also referred to as "cave cotton" when in its fibrous form. Occurrence Epsomite forms as encrustations or efflorescences on limestone caver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal Vein (geology), veins and as secondary minerals in the Redox, oxidizing zone of sulfide mineral deposits. The Chromate ion, chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 **Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O **Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Goslarite
Goslarite is a water of crystallization, hydrated zinc sulfate mineral () which was first found in the Rammelsberg mine, Goslar, Harz, Germany. It was described in 1847. Goslarite belongs to the epsomite group which also includes epsomite () and morenosite (). Goslarite is an unstable mineral at the surface and will dehydrate to other minerals like bianchite (), boyleite () and gunningite (). Physical properties The composition of goslarite was determined by the US National Bureau of Standards (now the National Institute of Standards and Technology) in 1959 as follows: SO3 27.84 wt%, ZnO 28.30 wt% and 43.86 wt%. Goslarite's cleavage is perfect in , as for epsomite and morenosite. The color of goslarite ranges from brownish to pinkish, blue, brown, colorless, green and green blue. The luster ranges from vitreous to nacreous and silky (if fibrous). Goslarite is soluble in water, has an astringent taste, and is strongly diamagnetic. Geologic occurrence Goslarite is formed from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pickeringite
Pickeringite is a magnesium aluminium sulfate mineral with formula MgAl2(SO4)4·22(H2O). It forms a series with halotrichite. It forms as an alteration product of pyrite in aluminium rich rocks and in coal seams. It also occurs in pyrite rich hydrothermal ore deposits in arid regions. It forms in fumaroles and in caves. It occurs with kalinite, alunogen, epsomite, melanterite, copiapite and gypsum. It was first described in 1844 as reflective for an occurrence in Cerros Pintados, Pampa del Tamarugal, Iquique Province, Tarapacá Region, Chile Chile, officially the Republic of Chile, is a country in western South America. It is the southernmost country in the world and the closest to Antarctica, stretching along a narrow strip of land between the Andes, Andes Mountains and the Paci .... It was named for American linguist and philologist John Pickering (1777–1846). References Sulfate minerals Monoclinic minerals Minerals in space group 14 {{sulfate-mineral-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halotrichite
Halotrichite, also known as feather alum, is a highly hydrated sulfate of aluminium and iron. Its chemical formula is . It forms fibrous monoclinic crystals. The crystals are water-soluble. It is formed by the weathering and decomposition of pyrite commonly near or in volcanic vents. The locations of natural occurrences include: the Atacama Desert, Chile; Dresden in Saxony, Germany; San Juan County, Utah; Iceland; Idrija, Slovenia; and Mont Saint-Hilaire, Canada. The name is from Latin: ''halotrichum'' for salt hair which accurately describes the precipitate/evaporite mineral. ;Gallery File:Halotrichite Hydrous iron aluminum sulfate Corral, California 3009.jpg, Halotrichite from California California () is a U.S. state, state in the Western United States that lies on the West Coast of the United States, Pacific Coast. It borders Oregon to the north, Nevada and Arizona to the east, and shares Mexico–United States border, an ... File:Halotrichite-179634.jpg, Halotrich ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also Crystallization, crystallizes as translucent crystals of selenite (mineral), selenite. It forms as an evaporite mineral and as a Mineral hydration, hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on Scratch hardness, scratch hardness comparison. Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Etymology and history The word ''wikt:gypsum, gypsum'' is derived from the Greek language, Greek word (), "plaster". Because the quarry, quarries of the Montmartre district of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanterite
Melanterite is a mineral form of hydrous iron(II) sulfate: FeSO4·7H2O. It is the iron analogue of the copper sulfate chalcanthite. It alters to siderotil by Dehydration, loss of water. It is a secondary sulfate mineral which forms from the oxidation of primary sulfide minerals such as pyrite and marcasite in the near-surface environment. It often occurs as a ''post mine'' encrustation on old underground mine surfaces. It also occurs in coal and lignite seams exposed to humid air and as a rare Volcanic sublimate, sublimate phase around volcanic fumaroles. Associated minerals include pisanite, chalcanthite, epsomite, pickeringite, halotrichite and other sulfate minerals. It was first described in 1850. Gallery File:Melanterite crystal structure.png, Crystal structure of melanterite File:Cuprian Melanterite - Parys Mountain Mines, Amlwch, Isle of Anglesey, Wales, UK.jpg, Cuprian melanterite References Sulfate minerals Iron(II) minerals Monoclinic minerals Minerals in spa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean deposits, and non-marine, which are found in standing bodies of water such as lakes. Evaporites are considered sedimentary rocks and are formed by chemical sediments. Formation Although all water bodies on the surface and in aquifers contain dissolved salts, the water must evaporate into the atmosphere for the minerals to precipitate. For this to happen, the water body must enter a restricted environment where water input into this environment remains below the net rate of evaporation. This is usually an arid environment with a small drainage basin fed by a limited input of water. When evaporation occurs, the remaining water is enriched in salts, and they precipitate after the water becomes saturated. Depositional environments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or another rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcanic activity, but fumarole activity can also precede a volcanic eruption and has been used for eruption prediction. Most fumaroles die down within a few days or weeks of the end of an eruption, but a few are persistent, lasting for decades or longer. An area containing fumaroles is known as a fumarole field. The predominant vapor emitted by fumaroles is steam, formed by the circulation of groundwater through heated rock. This is typically accompanied by volcanic gases given off by magma cooling deep below the surface. These volcanic gases include sulfur compounds, such as various sulfur oxides and hydrogen sulfide, and sometimes hydrogen chloride, hydrogen fluoride, and other gases. A fumarole that emits significant sulfur compounds is some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volcanic
A volcano is commonly defined as a vent or fissure in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface. On Earth, volcanoes are most often found where tectonic plates are diverging or converging, and because most of Earth's plate boundaries are underwater, most volcanoes are found underwater. For example, a mid-ocean ridge, such as the Mid-Atlantic Ridge, has volcanoes caused by divergent tectonic plates whereas the Pacific Ring of Fire has volcanoes caused by convergent tectonic plates. Volcanoes resulting from divergent tectonic activity are usually non-explosive whereas those resulting from convergent tectonic activity cause violent eruptions."Mid-ocean ridge tectonics, volcanism and geomorphology." Geology 26, no. 455 (2001): 458. https://macdonald.faculty.geol.ucsb.edu/papers/Macdonald%20Mid-Ocean%20Ridge%20Tectonics.pdf Volcanoes can also form where there is stretching a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science), crystal forms of calcium carbonate . Limestone forms when these minerals Precipitation (chemistry), precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly Dolomite (rock), dolomite, a closely related rock, which contains a high percentage of the mineral Dolomite (mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efflorescence
In chemistry, efflorescence (Derived from the Latin verb 'efflorescere' roughly meaning 'to flower') is the migration of a salt to the surface of a porous material, where it forms a coating. The essential process involves the dissolving of an internally held salt in water or occasionally, in another solvent. The water, with the salt now held in solution, migrates to the surface, then evaporates, leaving a coating of the salt. In what has been described as "primary efflorescence", the water is the invader and the salt was already present internally, and a reverse process, where the salt is originally present externally and is then carried inside in solution, is referred to as "secondary efflorescence". Efflorescences can occur in natural and built environments. On porous construction materials it may present a cosmetic outer problem only (primary efflorescence causing staining), but can sometimes indicate internal structural weakness (migration/degradation of component materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |