|
Elongation Factor P
EF-P (elongation factor P) is an essential protein that in bacteria stimulates the formation of the first peptide bonds in protein synthesis. Studies show that EF-P prevents ribosomes from stalling during the synthesis of proteins containing consecutive prolines. EF-P binds to a site located between the binding site for the peptidyl tRNA ( P site) and the exiting tRNA ( E site). It spans both ribosomal subunits with its amino-terminal domain positioned adjacent to the aminoacyl acceptor stem and its carboxyl-terminal domain positioned next to the anticodon stem-loop of the P site-bound initiator tRNA. The EF-P protein shape and size is very similar to a tRNA and interacts with the ribosome via the exit “E” site on the 30S subunit and the peptidyl-transferase center (PTC) of the 50S subunit. EF-P is a translation aspect of an unknown function, therefore It probably functions indirectly by altering the affinity of the ribosome for aminoacyl-tRNA, thus increasing their reactivity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EIF-5A
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the ''EIF5A'' gene. It is the only known protein to contain the unusual amino acid hypusine 'N''ε-(4-amino-2-hydroxybutyl)-lysine which is synthesized on eIF5A at a specific lysine residue from the polyamine spermidine by two catalytic steps. EF-P is the bacterial homolog of eIF5A, which is modified post-translationally in a similar but distinct way. Both proteins are believed to catalyze peptide bond formation and help resolve ribosomal stalls, making them elongation factors despite the "initiation factor" name originally assigned. Faundes-Banka syndrome Germline deleterious heterozygous ''EIF5A'' variants cause Faundes-Banka syndrome. This rare human disorder is characterized by variable combinations of developmental delay, microcephaly, micrognathia and dysmorphic features. It was named after Víctor Faundes and Siddharth Banka. See also EIF5A2 Eukaryotic translation initiation f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In biology, translation is the process in living Cell (biology), cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in the addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the cell. The polypeptide can also start folding during protein synthesis. The ribosome facilitates decoding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EIF5A
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the ''EIF5A'' gene. It is the only known protein to contain the unusual amino acid hypusine 'N''ε-(4-amino-2-hydroxybutyl)-lysine which is synthesized on eIF5A at a specific lysine residue from the polyamine spermidine by two catalytic steps. EF-P is the bacterial homolog of eIF5A, which is modified post-translationally in a similar but distinct way. Both proteins are believed to catalyze peptide bond formation and help resolve ribosomal stalls, making them elongation factors despite the "initiation factor" name originally assigned. Faundes-Banka syndrome Germline deleterious heterozygous ''EIF5A'' variants cause Faundes-Banka syndrome. This rare human disorder is characterized by variable combinations of developmental delay, microcephaly Microcephaly (from Neo-Latin ''microcephalia'', from Ancient Greek μικρός ''mikrós'' "small" and κεφαλή ''kephalé'' "head") is a me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF-G
EF-G (elongation factor G, historically known as translocase) is a prokaryotic elongation factor involved in mRNA translation. As a GTPase, EF-G catalyzes the movement (translocation) of transfer RNA (tRNA) and messenger RNA (mRNA) through the ribosome. Structure Encoded by the ''fusA'' gene on the ''str'' operon, EF-G is made up of 704 amino acids that form 5 domains, labeled Domain I through Domain V. Domain I may be referred to as the G-domain or as Domain I(G), since it binds to and hydrolyzes guanosine triphosphate (GTP). Domain I also helps EF-G bind to the ribosome, and contains the N-terminal of the polypeptide chain. Domain IV is important for translocation, as it undergoes a significant conformational change and enters the A site on the 30S ribosomal subunit, pushing the mRNA and tRNA molecules from the A site to the P site. The five domains may be also separated into two super-domains. Super-domain I consists of Domains I and II, and super-domain II consists of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
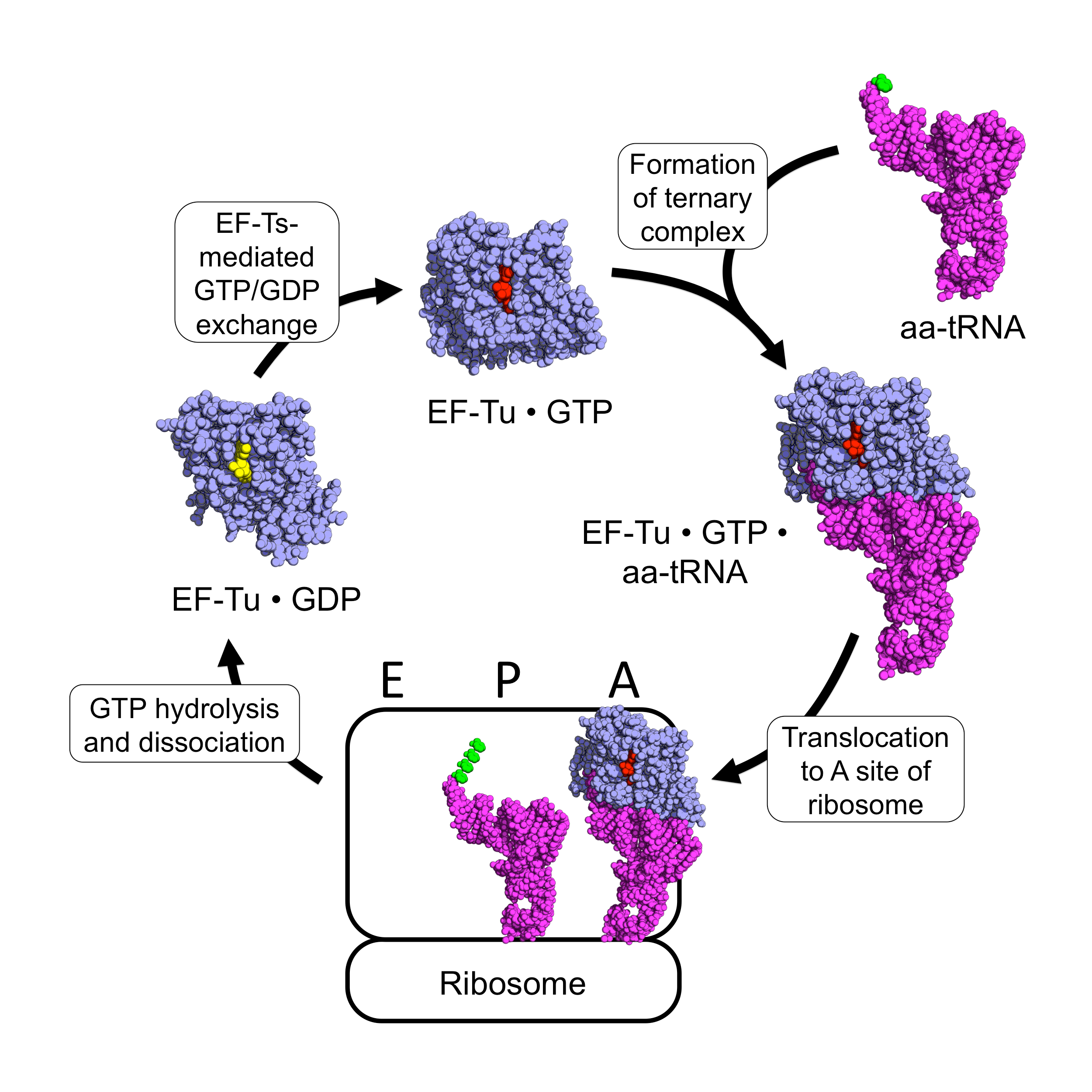
EF-Tu
EF-Tu (elongation factor thermo unstable) is a prokaryotic elongation factor responsible for catalyzing the binding of an aminoacyl-tRNA (aa-tRNA) to the ribosome. It is a G-protein, and facilitates the selection and binding of an aa-tRNA to the A-site of the ribosome. As a reflection of its crucial role in translation, EF-Tu is one of the most abundant and highly conserved proteins in prokaryotes. It is found in eukaryotic mitochondria as TUFM. As a family of elongation factors, EF-Tu also includes its eukaryotic and archaeal homolog, the alpha subunit of eEF-1 (EF-1A). Background Elongation factors are part of the mechanism that synthesizes new proteins through translation in the ribosome. Transfer RNAs (tRNAs) carry the individual amino acids that become integrated into a protein sequence, and have an anticodon for the specific amino acid that they are charged with. Messenger RNA (mRNA) carries the genetic information that encodes the primary structure of a protein, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF-Ts
EF-Ts (elongation factor thermo stable) is one of the prokaryotic elongation factors. It is found in human mitochondria as TSFM. It is similar to eukaryotic eEF-1, EF-1B. EF-Ts serves as the guanine nucleotide exchange factor for EF-Tu (elongation factor thermo unstable), catalyzing the release of guanosine diphosphate from EF-Tu. This enables EF-Tu to bind to a new guanosine triphosphate molecule, release EF-Ts, and go on to catalyze another aminoacyl tRNA addition. Structure The protein Qβ-Replicase is a tetrameric protein, meaning it contains four subunits. These subunits are the two elongation factors, EF-Tu & EF-Ts, the ribosomal protein subunit S1, and the RNA dependent RNA polymerase β-subunit. The two elongation factors form a heterodimer structure known as the elongation factor complex, which is necessary for the polymerization activity of the RDRP β-Subunit. Its secondary structural components consists of α-helices, β-sheets and β-barrels. EF-Ts comprises th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryotic Elongation Factors
Elongation factors are a set of proteins that function at the ribosome, during protein synthesis, to facilitate translational elongation from the formation of the first to the last peptide bond of a growing polypeptide. Most common elongation factors in prokaryotes are EF-Tu, EF-Ts, EF-G. Bacteria and eukaryotes use elongation factors that are largely homologous to each other, but with distinct structures and different research nomenclatures. Elongation is the most rapid step in translation. In bacteria, it proceeds at a rate of 15 to 20 amino acids added per second (about 45-60 nucleotides per second). In eukaryotes the rate is about two amino acids per second (about 6 nucleotides read per second). Elongation factors play a role in orchestrating the events of this process, and in ensuring the high accuracy translation at these speeds. Nomenclature of homologous EFs In addition to their cytoplasmic machinery, eukaryotic mitochondria and plastids have their own translation m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Puromycin
Puromycin is an antibiotic protein synthesis inhibitor which causes premature chain termination during translation. Inhibition of translation Puromycin is an aminonucleoside antibiotic, derived from the '' Streptomyces alboniger'' bacterium, that causes premature chain termination during translation taking place in the ribosome. Part of the molecule resembles the 3' end of the aminoacylated tRNA. It enters the A site and transfers to the growing chain, causing the formation of a puromycylated nascent chain and premature chain release. The exact mechanism of action is unknown at this time but the 3' position contains an amide linkage instead of the normal ester linkage of tRNA. That makes the molecule much more resistant to hydrolysis and stops the ribosome. Puromycin is selective for either prokaryotes or eukaryotes. Also of note, puromycin is critical in mRNA display. In this reaction, a puromycin molecule is chemically attached to the end of an mRNA template, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Termination Factor
In molecular biology, a termination factor is a protein that mediates the termination of RNA transcription by recognizing a transcription terminator and causing the release of the newly made mRNA. This is part of the process that regulates the transcription of RNA to preserve gene expression integrity and are present in both eukaryotes and prokaryotes, although the process in bacteria is more widely understood. The most extensively studied and detailed transcriptional termination factor is the Rho (ρ) protein of ''E. coli''. Prokaryotic Prokaryotes use one type of RNA polymerase, transcribing mRNAs that code for more than one type of protein. Transcription, translation and mRNA degradation all happen simultaneously. Transcription termination is essential to define boundaries in transcriptional units, a function necessary to maintain the integrity of the strands and provide quality control. Termination in ''E. coli'' may be Rho dependent, utilizing Rho factor, or Rho independen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elongation Factors
Elongation may refer to: * Elongation (astronomy) * Elongation (geometry) * Elongation (plasma physics) * Part of transcription of DNA into RNA of all types, including mRNA, tRNA, rRNA, etc. * Part of translation (biology) of mRNA into proteins * Elongated organisms * Elongation (mechanics) In physics and continuum mechanics, deformation is the change in the shape or size of an object. It has dimension of length with SI unit of metre (m). It is quantified as the residual displacement of particles in a non- rigid body, from an con ..., linear deformation See also * {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Initiation Factors
In molecular biology, initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis. Initiation factors can interact with repressors to slow down or prevent translation. They have the ability to interact with activators to help them start or increase the rate of translation. In bacteria, they are simply called IFs (i.e.., IF1, IF2, & IF3) and in eukaryotes they are known as eIFs (i.e.., eIF1, eIF2, eIF3). Translation initiation is sometimes described as three step process which initiation factors help to carry out. First, the tRNA carrying a methionine amino acid binds to the small subunit of ribosome, then binds to the mRNA, and finally joins together with the large subunit of ribosome. The initiation factors that help with this process each have different roles and structures. Types The initiation factors are divided into three major groups by taxonomic domains. There are some homologies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |