|
Effective Porosity
Effective porosity is most commonly considered to represent the porosity of a rock or sediment available to contribute to fluid flow through the rock or sediment, or often in terms of "flow to a borehole". Porosity that is not considered "effective porosity" includes water bound to clay particles (known as bound water) and isolated "vuggy" porosity (vugs not connected to other pores, or dead-end pores). The effective porosity is of great importance in considering the suitability of rocks or sediments as oil or gas reservoirs, or as aquifers. The term lacks a single or straightforward definition. Even some of the terms used in its mathematical description ("V_” and “V_”) have multiple definitions. Background for multiple definitions Quartz "Quartz" (more aptly termed “non-clay minerals”) forms part of the matrix, or in core analysis terms, part of the grain volume. Clay layers "Clay layers" are dry clay (Vcl) which also form part of the grain volume. If a core samp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, rock mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, , the chief constituent of limestone (as well as the main component of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smectite
A smectite (; ; ) is a mineral mixture of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secondary minerals such as quartz and calcite. Terminology In clay mineralogy, smectite is synonym of montmorillonite (also the name of a pure clay mineral phase) to indicate a class of swelling clays. The term smectite is commonly used in Europe and in the UK while the term montmorillonite is preferred in North America, but both terms are equivalent and can be used interchangeably. For industrial and commercial applications, the term bentonite is mostly used in place of smectite or montmorillonite. Mineralogical structure The 2:1 layer (TOT) structure consists of two Silicon dioxide, silica (SiO2) Tetrahedral molecular geometry, tetrahedral (T) layers which are electrostatically cross-linked via an Al2O3 (gibbsite), or Fe2O3, Octahedral mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect basal cleavage''. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites. Micas are used in products such as drywalls, paints, and fillers, especially in parts for automobiles, roofing, and in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost". Properties and structure The mica group comprises 37 phyllosilicate minerals. All crystallize in the monoclinic system, with a tendency towards pseudohexagonal crystals, and are similar in structure but vary in chemical composition. Micas are translucent to opa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Illite
Illite, also called hydromica or hydromuscovite, is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina octahedron (O) – silica tetrahedron (T) layers. The space between this T-O-T sequence of layers is occupied by poorly hydrated potassium cations which are responsible for the absence of swelling. Structurally, illite is quite similar to muscovite with slightly more silicon, magnesium, iron, and water and slightly less tetrahedral aluminium and interlayer potassium. The chemical formula is given as , but there is considerable ion (isomorphic) substitution. It occurs as aggregates of small monoclinic grey to white crystals. Due to the small size, positive identification usually requires x-ray diffraction or SEM-EDS ( automated mineralogy) analysis. Illite occurs as an altered product of muscovite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glauconite
Glauconite is an iron potassium phyllosilicate ( mica group) mineral of characteristic green color which is very friable and has very low weathering resistance. It crystallizes with a monoclinic geometry. Its name is derived from the Greek () meaning 'bluish green', referring to the common blue-green color of the mineral; its sheen ( mica glimmer) and blue-green color. Its color ranges from olive green, black green to bluish green, and yellowish on exposed surfaces due to oxidation. In the Mohs scale it has a hardness of 2, roughly the same as gypsum. The relative specific gravity range is 2.4–2.95. It is normally found as dark green rounded concretions with the dimensions of a sand grain. It can be confused with chlorite (also of green color) or with a clay mineral. Glauconite has the chemical formula . Glauconite particles are one of the main components of greensand, glauconitic siltstone and glauconitic sandstone. Glauconite has been called a marl in an old and br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Western Australia
Western Australia (WA) is the westernmost state of Australia. It is bounded by the Indian Ocean to the north and west, the Southern Ocean to the south, the Northern Territory to the north-east, and South Australia to the south-east. Western Australia is Australia's largest state, with a land area of , and is also the List of country subdivisions by area, second-largest subdivision of any country on Earth. Western Australia has a diverse range of climates, including tropical conditions in the Kimberley (Western Australia), Kimberley, deserts in the interior (including the Great Sandy Desert, Little Sandy Desert, Gibson Desert, and Great Victoria Desert) and a Mediterranean climate on the south-west and southern coastal areas. the state has 2.965 million inhabitants—10.9 percent of the national total. Over 90 percent of the state's population live in the South-West Land Division, south-west corner and around 80 percent live in the state capital Perth, leaving the remainder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greensand (geology)
Greensand or green sand is a sand or sandstone which has a greenish color. This term is specifically applied to shallow marine sediment that contains noticeable quantities of rounded greenish grains. These grains are called ''glauconies'' and consist of a mixture of mixed-layer clay minerals, such as smectite and glauconite. Greensand is also loosely applied to any glauconitic sediment. Formation Greensand forms in Anoxic sea water, anoxic Marine (ocean), marine environments that are rich in Organic matter, organic detritus and low in sedimentary input. Having accumulated in marine environments, greensands can be fossil-rich, such as in the late-Cretaceous deposits of New Jersey, United States. Occurrence Important exposures are known from both northern and western Europe, North America, Southeast Region, Brazil, southeastern Brazil and north Africa. Well known and important greensands are the Upper Greensand Formation, Upper and Lower Greensand Group, Lower Greensands of Englan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Litre
The litre ( Commonwealth spelling) or liter ( American spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre (or litre) occupies a volume of (see figure) and is thus equal to one-thousandth of a cubic metre. The original French metric system used the litre as a base unit. The word ''litre'' is derived from an older French unit, the '' litron'', whose name came from Byzantine Greek—where it was a unit of weight, not volume—via Late Medieval Latin, and which equalled approximately 0.831 litres. The litre was also used in several subsequent versions of the metric system and is accepted for use with the SI, despite it not being an SI unit The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of unit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one thousandth of a kilogram. Originally defined in 1795 as "the absolute Mass versus weight, weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (0 °C) was later changed to the temperature of maximum density of water (approximately 4 °C). Subsequent redefinitions agree with this original definition to within 30 Parts-per notation, parts per million (0.003%), with the maximum density of water remaining very close to 1 g/cm3, as shown by modern measurements. By the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation Exchange Capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with other positively charged particles in the surrounding soil water. This is one of the ways that solid materials in soil alter the chemistry of the soil. CEC affects many aspects of soil chemistry, and is used as a measure of soil fertility, as it indicates the capacity of the soil to retain several nutrients (e.g. K+, NH4+, Ca2+) in plant-available form. It also indicates the capacity to retain pollutant cations (e.g. Pb2+). Definition and principles Cation-exchange capacity is defined as the amount of positive charge that can be exchanged per mass of soil, usually measured in cmolc/kg. Some texts use the older, equivalent units me/100g or meq/100g. CEC is measured in moles of electric charge, so a cation-exchange capacity of 10 cmolc/kg co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
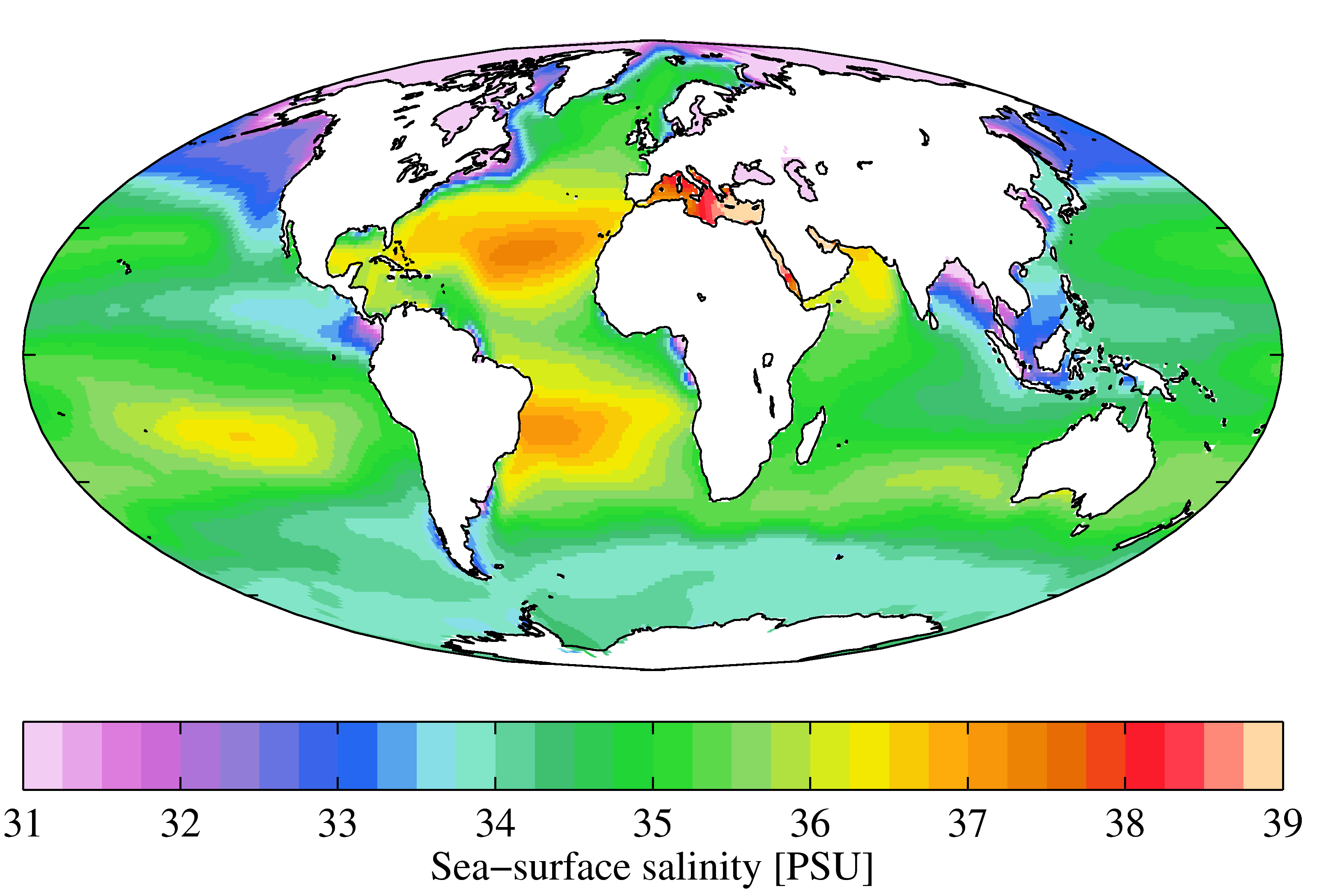
Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to per mille, ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a state function, thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |