|
EVA Foam
Ethylene-vinyl acetate (EVA), also known as poly(ethylene-vinyl acetate) (PEVA), is a copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 50%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used. The EVA copolymer which is based on a low proportion of VA (approximately up to 4%) may be referred to as vinyl acetate modified polyethylene. It is a copolymer and is processed as a thermoplastic material – just like low-density polyethylene. It has some of the properties of a low-density polyethylene but increased gloss (useful for film), softness and flexibility. The material is generally considered non-toxic. The EVA copolymer which is based on a medium proportion of VA (approximately 4 to 30%) is referred to as thermoplastic ethylene-vinyl acetate copolymer and is a thermoplastic elastomer material. It is not vulcan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eva Closeup
Eva or EVA may refer to: * Eva (name), a feminine given name Arts, entertainment, and media Fictional characters * Eva (Dynamite Entertainment), a comic book character * Eva (Devil May Cry), Eva (''Devil May Cry''), in the ''Devil May Cry'' video game series * Eva (Metal Gear), Eva (''Metal Gear''), in the ''Metal Gear'' video games series * Eva Mapendo, in 2018 romantic drama series ''Ngayon at Kailanman (2018 TV series), Ngayon at Kailanman'', portrayed by Julia Barretto * Evangelion (mecha), in the ''Neon Genesis Evangelion'' franchise Films * Eva (1948 film), ''Eva'' (1948 film), a Swedish film * Eva (1953 film), ''Eva'' (1953 film), a Greek drama film * Eva (1958 film), ''Eva'' (1958 film), an Austrian film * Eva (1962 film), ''Eva'' (1962 film), a French-Italian film in English * Eva (2010 film), ''Eva'' (2010 film), an English-language Romanian film * Eva (2011 film), ''Eva'' (2011 film), a Spanish film * Eva (2018 film), ''Eva'' (2018 film), a French film * Eva (2023 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flutterboard
A swimming float, commonly known as pool float or floaty, is a device used for toddlers or other very young children who are beginning to learn how to swim, or during exercise for therapeutic or training purposes. These devices, which come in many shapes and types, are used to aid them with buoyancy, or for floating on for fun. The most common floats for children and adults are inflatable rings (in the middle of which the user swims) and inflatable armbands (placed around the user's arms). After being inflated through a valve, they are much less dense than water because they are composed mainly of air, surrounded by a thin layer of synthetic material. Float-assisted swimming can be more difficult than free swimming, because if the float is held in front of the swimmer a more vigorous workout for the legs is given as the swimmer's weight is propelled solely by the legs, and vice versa for the arms. Swimming board Swimming boards (often referred to as kickboards or flutter bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pool Noodle
A pool noodle is a cylindrical piece of flexible, buoyant polyethylene foam. Pool noodles are used by people of all ages while swimming. Pool noodles are useful when learning to Swimming, swim, for floating, for rescue reaching, in various forms of water play, and for aquatic exercise. The most common dimensions are about in length and in diameter. The pool noodle is also used for people who experience difficulties in swimming. The pool noodle is often used to protect sharp edges and corners. Types The term "water woggle" derives from Koswell Holdings trademark Water Woggle, which was first marketed as a foam water toy in the 1980s. The term "noodle" derives from Jakks Pacific's trademark FunNoodle water product, which was created as a foam tube water toy. Canoodle ("connect a noodle") is the polypropylene (plastic) erector set manufactured in the US by Serranoventions. Connectors There are several pool noodle connectors on the market. One connector is a piece of pip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Vinyl Alcohol
Ethylene vinyl alcohol (EVOH) is a formal copolymer of ethylene and vinyl alcohol. Because the latter monomer mainly exists as its tautomer acetaldehyde, the copolymer is prepared by polymerization of ethylene and vinyl acetate to give the ethylene vinyl acetate (EVA) copolymer followed by hydrolysis. EVOH copolymer is defined by the mole % ethylene content: lower ethylene content grades have higher barrier properties; higher ethylene content grades have lower temperatures for extrusion. The plastic resin is commonly used as an oxygen barrier in food packaging. It is better than other plastics at keeping air out and flavors in, is highly transparent, weather resistant, oil and solvent resistant, flexible, moldable, recyclable, and printable. Its drawback is that it is difficult to make and therefore more expensive than other food packaging. Instead of making an entire package out of EVOH, manufacturers keep costs down by coextruding or laminating it as a thin layer between c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud, crystals in a rock, or polymer macromolecules in a solution or a solid polymer mass. Polymers can be described by molecular mass distribution; a population of particles can be described by size, surface area, and/or mass distribution; and thin films can be described by film thickness distribution. IUPAC has deprecated the use of the term ''polydispersity index'', having replaced it with the term ''dispersity'', represented by the symbol Đ (pronounced D-strokeStepto, R. F. T.; Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl P. (2009).Dispersity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain Transfer
In polymer chemistry, chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule: \ce^\bullet + \ce^\bullet where • is the active center, P is the initial polymer chain, X is the end group, and R is the substituent to which the active center is transferred. Chain transfer reactions reduce the average molecular weight of the final polymer. Chain transfer can be either introduced deliberately into a polymerization (by use of a ''chain transfer agent'') or it may be an unavoidable side-reaction with various components of the polymerization. Chain transfer reactions occur in most forms of addition polymerization including radical polymerization, ring-opening polymerization, coordination polymerization, and cationic polymerization, as well as anionic polymerization. Types Chain transfer reactions are usually categorized by the nature of the molecule that reacts with the growing chain. * Transfer to cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
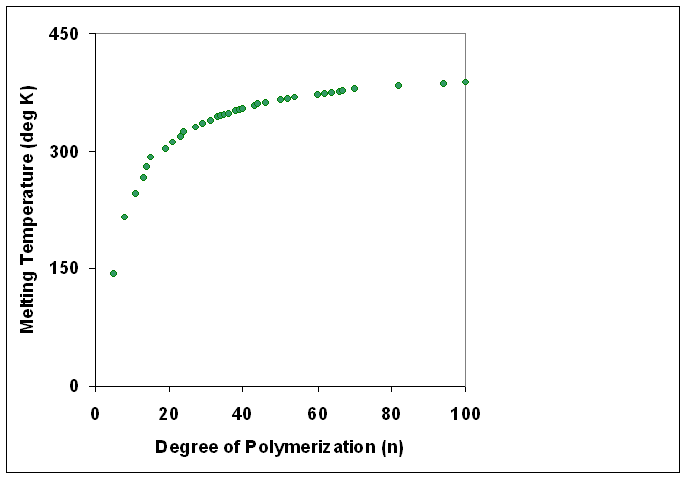
Degree Of Polymerization
The degree of polymerization, or DP, is the number of structural unit, monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by \overline_n\equiv\overline_n=\frac, where \overline_n is the Molar mass distribution#Number average molar mass, number-average molecular weight and M_0 is the molecular weight of the monomer unit. The overlines indicate arithmetic mean values. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units. Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per metre squared, or pascal-seconds. Viscosity quantifies the internal friction, frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's center line than near its walls. Experiments show that some stress (physics), stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and to adjacent elements through Single bond, single covalent bonds, denoted by dashes or lines. The group in a peroxide is often called the peroxide group, though some nomenclature discrepancies exist. This linkage is recognized as a common polyatomic ion, and exists in many molecules. General structure The characteristic structure of any regular peroxide is the oxygen–oxygen covalent single bond, which connects the two main atoms together. In the event that the molecule has no chemical Substituent, substituents, the peroxide group will have a [−2] Formal charge, net charge. Each oxygen atom has a charge of negative one, as 5 of its Valence electron, valence electrons remain in the outermost Atomic orbital, orbital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |