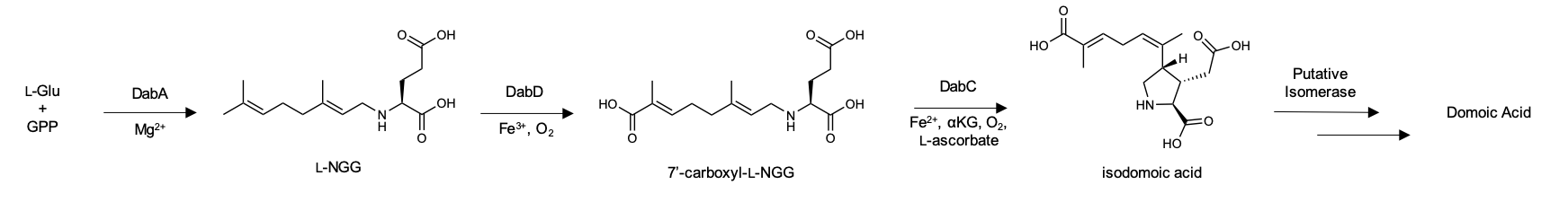
|
Domoic Acid
Domoic acid (DA) is a kainic acid-type neurotoxin that causes amnesic shellfish poisoning (ASP). It is produced by algae and accumulates in shellfish, sardines, and anchovies. When sea lions, otters, cetaceans, humans, and other predators eat contaminated animals, poisoning may result. Exposure to this compound affects the brain, causing seizures, delirium and possibly death. History There has been little use of domoic acid throughout history except for in Japan, where it has been used as an anthelmintic for centuries. Domoic acid was first isolated in 1959 from a species of red algae, ''Chondria (alga), Chondria armata'', in Japan, which is commonly referred to as ''dōmoi'' (ドウモイ) in the Tokunoshima dialect, or ''hanayanagi''. Poisonings in history have been rare, or undocumented; however, it is thought that the increase in human activities is resulting in an increasing frequency of harmful algal blooms along coastlines in recent years. In 2015, the North American Pacific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kainic Acid
Kainic acid, or kainate, is an acid that naturally occurs in some seaweed. Kainic acid is a potent neuroexcitatory amino acid agonist that acts by activating receptors for glutamate, the principal excitatory neurotransmitter in the central nervous system. Glutamate is produced by the cell's metabolic processes and there are four major classifications of glutamate receptors: NMDA receptors, AMPA receptors, kainate receptors, and the metabotropic glutamate receptors. Kainic acid is an agonist for kainate receptors, a type of ionotropic glutamate receptor. Kainate receptors likely control a sodium channel that produces excitatory postsynaptic potentials (EPSPs) when glutamate binds. Kainic acid is commonly injected into laboratory animal models to study the effects of Ablative brain surgery, experimental ablation. Kainic acid is a direct agonist of the glutamic kainate receptors and large doses of concentrated solutions produce immediate neuronal death by overstimulating neurons to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
California Brown Pelican
The brown pelican (''Pelecanus occidentalis'') is a bird of the pelican family, Pelecanidae, one of three species found in the Americas and one of two that feed by diving into water. It is found on the Atlantic Coast from New Jersey to the mouth of the Amazon River, and along the Pacific coast, Pacific Coast from British Columbia to Peru, including the Galapagos Islands. The nominate subspecies in its Glossary of bird terms#breeding plumage, breeding plumage has a white head with a yellowish wash on the crown. The nape and neck are dark maroon–brown. The upper sides of the neck have white lines along the base of the gular pouch, and the lower fore neck has a pale yellowish patch. The male and female are similar, but the female is slightly smaller. The nonbreeding adult has a white head and neck. The pink skin around the eyes becomes dull and gray in the nonbreeding season. It lacks any red hue, and the pouch is strongly olivaceous ochre-tinged and the legs are olivaceous gra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excitotoxicity
In excitotoxicity, neuron, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamic acid, glutamate become pathologically high, resulting in excessive stimulation of cell surface receptor, receptors. For example, when glutamate receptors such as the NMDA receptor or AMPA receptor encounter excessive levels of the excitatory neurotransmitter, glutamate, significant neuronal damage might ensue. Different mechanisms might lead to increased extracellular glutamate concentrations, e.g. reduced uptake by glutamate transporters (EAATs), synaptic hyperactivity, or abnormal release from different neural cell types. Excess glutamate allows high levels of calcium in biology, calcium ions (Ca2+) to enter the cell (biology), cell. Ca2+ influx into cells activates a number of enzymes, including phospholipases, endonucleases, and proteases such as calpain. These enzymes go on to damage cell structures such as components of the cytoskel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate Receptors
Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate (the conjugate base of glutamic acid) is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation. Glutamate receptors are implicated in a number of neurological conditions. Their central role in excitotoxicity and prevalence in the central nervous system has been linked or speculated to be linked to many neurodegenerative diseases, and several other conditions have been further linked to glutamate receptor gene mutations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricarboxylic Acid
A tricarboxylic acid is an organic carboxylic acid that contain three carboxyl functional groups (−COOH). A well-known example is citric acid. Promient examples Some prominent substituted tricarboxylic acids Citric acid, is used in the citric acid cyclealso known as the ''tricarboxylic acid'' (''TCA'') ''cycle'' or ''Krebs cycle''which is fundamental to all aerobic organisms. Nitrilotriacetic acid (NTA) is a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+. See also * Citric acid cycle (tricarboxylic acid cycle) * Dicarboxylic acid In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarbox ... * Mellitic acid References Literature *{{cite journal , title = The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist. , author = Ryan J. Mailloux, Robin Bériaul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant '' S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (C), which makes up 99% of all carbon on Earth; carbon-13 (C), which makes up 1%; and carbon-14 (C), which occurs in trace amounts, making up about 1-1.5 atoms per 10 atoms of carbon in the atmosphere. C and C are both stable; C is unstable, with half-life years. Carbon-14 has a specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g. Carbon-14 decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the optical rotation, dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxylic acid, carboxyl groups −COOH and one amine, amino group � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |