|
Diammonium Phosphate
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4)) is one of a series of water- soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid. Solid diammonium phosphate shows a dissociation pressure of ammonia as given by the following expression and equation: : At 100 °C, the dissociation pressure of diammonium phosphate is approximately 5 mmHg. According to the diammonium phosphate MSDS from CF Industries, Inc., decomposition starts as low as 70 °C: "Hazardous Decomposition Products: Gradually loses ammonia when exposed to air at room temperature. Decomposes to ammonia and monoammonium phosphate at around 70 °C (158 °F). At 155 °C (311 °F), DAP emits phosphorus oxides, nitrogen oxides and ammonia." Uses DAP is used as a fertilizer. When applied as plant fertilizer, it temporarily increases the soil pH, but over a long term the treated ground becomes more acidic than before, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide * Nitrogen dioxide (), nitrogen(IV) oxide * Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide * Dinitrogen dioxide (), nitrogen(II) oxide dimer * Dinitrogen trioxide (), nitrogen(II,IV) oxide * Dinitrogen tetroxide (), nitrogen(IV) oxide dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate * Nitrosyl azide (), nitrogen(−I,0,I,II) oxide * Nitryl azide () * Oxatetrazole () * Trinitramide ( or ), nitrogen(0,IV) oxide Anions Cations * Nitrosonium ( or ) * Nitronium ( or ) Atmospheric sciences In atmospheric chemistry: * (or NO''x'') refers to the sum of NO and . * (or NO''y'') refers to the sum of and all oxidized atmospheric odd-nitrogen species (''e.g.'' the sum of , , , etc.) * (or NO''z'') = − * Mixed Oxides of Nitrogen ("MON"): solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
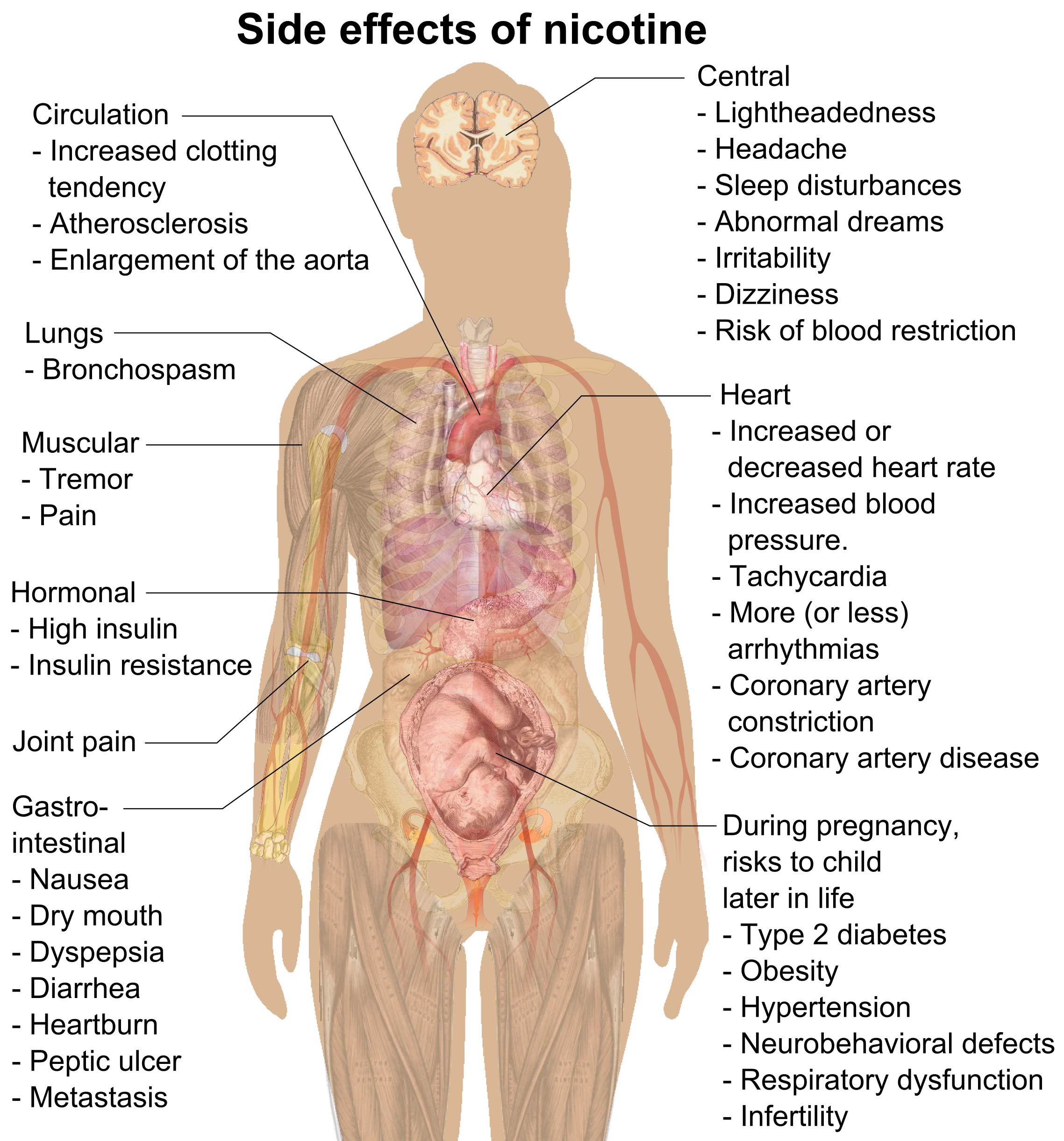
Nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve drug withdrawal, withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at Parts-per notation, ppb concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an plant defense against herbivory, antiherbivore toxin; consequently, nicotine was widely used as an insecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mead
Mead (), also called honey wine, and hydromel (particularly when low in alcohol content), is an alcoholic beverage made by fermenting honey mixed with water, and sometimes with added ingredients such as fruits, spices, grains, or hops. The alcoholic content ranges from about 3.5% ABV to more than 20%. Possibly the most ancient alcoholic drink, the defining characteristic of mead is that the majority of the beverage's fermentable sugar is derived from honey. It may be still, carbonated, or naturally sparkling, and despite a common misconception that mead is exclusively sweet, it can also be dry or semi-sweet. Mead that also contains spices is called (), and mead that contains fruit is called melomel. The term honey wine is sometimes used as a synonym for mead, although wine is typically defined to be the product of fermented grapes or certain other fruits, and some cultures have honey wines that are distinct from mead. The honey wine of Hungary, for example, is the fermentation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yeast Assimilable Nitrogen
Yeast assimilable nitrogen or YAN is the combination of free amino nitrogen (FAN), ammonia (NH3) and ammonium (NH4+) that is available for a yeast, e.g. the wine yeast ''Saccharomyces cerevisiae'', to use during fermentation (wine), fermentation. Outside of the fermentable sugars (wine), fermentable sugars glucose and fructose, nitrogen is the most important nutrient needed to carry out a successful fermentation that doesn't end prior to the intended point of dryness (wine), dryness or sees the development of off-odors and related wine faults. To this extent winemakers will often supplement the available YAN resources with nitrogen additives such as diammonium phosphate (DAP).B. Zoecklein, K. Fugelsang, B. Gump, F. Nury ''Wine Analysis and Production'' pgs 152-163, 340-343, 444-445, 467 Kluwer Academic Publishers, New York (1999) However, the addition of excessive amounts of nitrogen can also create a hazard as other organisms besides beneficial wine yeast can utilize the nutrien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Firebreak
A firebreak or double track (also called a fire line, fuel break, fireroad and firetrail in Australia) is a gap in vegetation or other combustible material that acts as a barrier to slow or stop the progress of a bushfire or wildfire. A firebreak may occur naturally where there is an absence of vegetation or "fuel", such as a river, lake or canyon. Firebreaks may also be man-made, and many of these also serve as roads, such as logging roads, four-wheel drive trails, secondary roads, or highways. Overview In the construction of a firebreak, the primary goal is to remove deadwood and undergrowth down to mineral soil. Various methods may be used to accomplish this initially and to maintain this condition. Ideally, the firebreak will be constructed and maintained according to the established practices of sustainable forestry and fire protection engineering, also known as best management practices (BMP). The general goals are to maximize the effectiveness of the firebreak at slowin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Char (chemistry)
Char is the solid material that remains after light gases (e.g. coal gas) and tar have been driven out or released from a carbonaceous material during the initial stage of combustion, which is known as carbonization, charring, devolatilization or pyrolysis. Further stages of efficient combustion (with or without char deposits) are known as gasification reactions, ending quickly when the reversible gas phase of the water gas shift reaction is reached. See also * Biochar * Charcoal * Coke (fuel) * Petroleum coke Petroleum coke, abbreviated coke, pet coke or petcoke, is a final carbon-rich solid material that derives from oil refinery, oil refining, and is one type of the group of fuels referred to as Coke (fuel), cokes. Petcoke is the coke that, in parti ... * Shale oil extraction * Spent shale References Oil shale Coal {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wildfires
A wildfire, forest fire, or a bushfire is an unplanned and uncontrolled fire in an area of Combustibility and flammability, combustible vegetation. Depending on the type of vegetation present, a wildfire may be more specifically identified as a bushfire (Bushfires in Australia, in Australia), desert fire, grass fire, hill fire, Peat#Peat fires, peat fire, prairie fire, vegetation fire, or veld fire. Some natural forest ecosystems Fire ecology, depend on wildfire. Modern forest management often engages in prescribed burns to mitigate fire risk and promote natural forest cycles. However, controlled burns can turn into wildfires by mistake. Wildfires can be classified by cause of ignition, physical properties, combustible material present, and the effect of weather on the fire. Wildfire severity results from a combination of factors such as available fuels, physical setting, and weather. Climatic cycles with wet periods that create substantial fuels, followed by drought and heat, of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are commonly used in fire fighting, where they may be applied aerially or from the ground. Principles of operation In general, fire retardants reduce the flammability of materials by either blocking the fire physically or by initiating a chemical reaction that stops the fire. Physical action There are several ways in which the combustion process can be retarded by physical action: * By cooling: Some chemical reactions actually cool the material down. * By forming a protective layer that prevents the underlying material from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |