|
Denigés' Reagent
The Denigés' reagent is a reagent used for qualitative analysis. It was developed in 1898 by Georges Denigés (December 25, 1859–February 20, 1951), a French biochemist. Uses Denigés' reagent is used to detect isolefin or tertiary alcohols which can be easily dehydrated to form isoolefin in the presence of acid. Treatment of solutions containing either isolefin or tertiary alcohols with this reagent will result in the formation of a solid yellow or red precipitate. Synthesis Despite the different stoichiometry in these mixtures which varies the concentration of the reagent, they all follow the same idea of adding HgO to distilled water and concentrated sulfuric acid. The Denigés' reagent is ultimately mercury(II) sulfate in an aqueous solution. *5 grams of mercury(II) oxide (HgO) is dissolved in 40 mL of distilled water. The mixture is slowly stirred, while 20 mL of concentrated sulfuric acid is added. After adding another 40 mL of distilled water, the solution is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualitative Inorganic Analysis
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes. Qualitative inorganic analysis is that branch or method of analytical chemistry which seeks to establish the elemental composition of inorganic compounds through various reagents. Physical appearance of inorganic salts Detecting cations According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution. To obtain meaningful results, the separation must be done in the sequence specified below, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Alcohol
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Dehydration reactions in organic chemistry Esterification The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol to give an ester :RCO2H + R′OH RCO2R′ + H2O Often such reactions require the presence of a dehydrating agent, i.e. a substance that reacts with water. Etherification Two monosaccharides, such as glucose and fructose, can be joined together (to form saccharose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide. Nitrile formation Nitriles are often prepared by dehydration of primary amides. :RC(O)NH2 → RCN + H2O Ketene formation Ketene is produced by heating acetic acid and trapping the product: :CH3CO2H → CH2= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
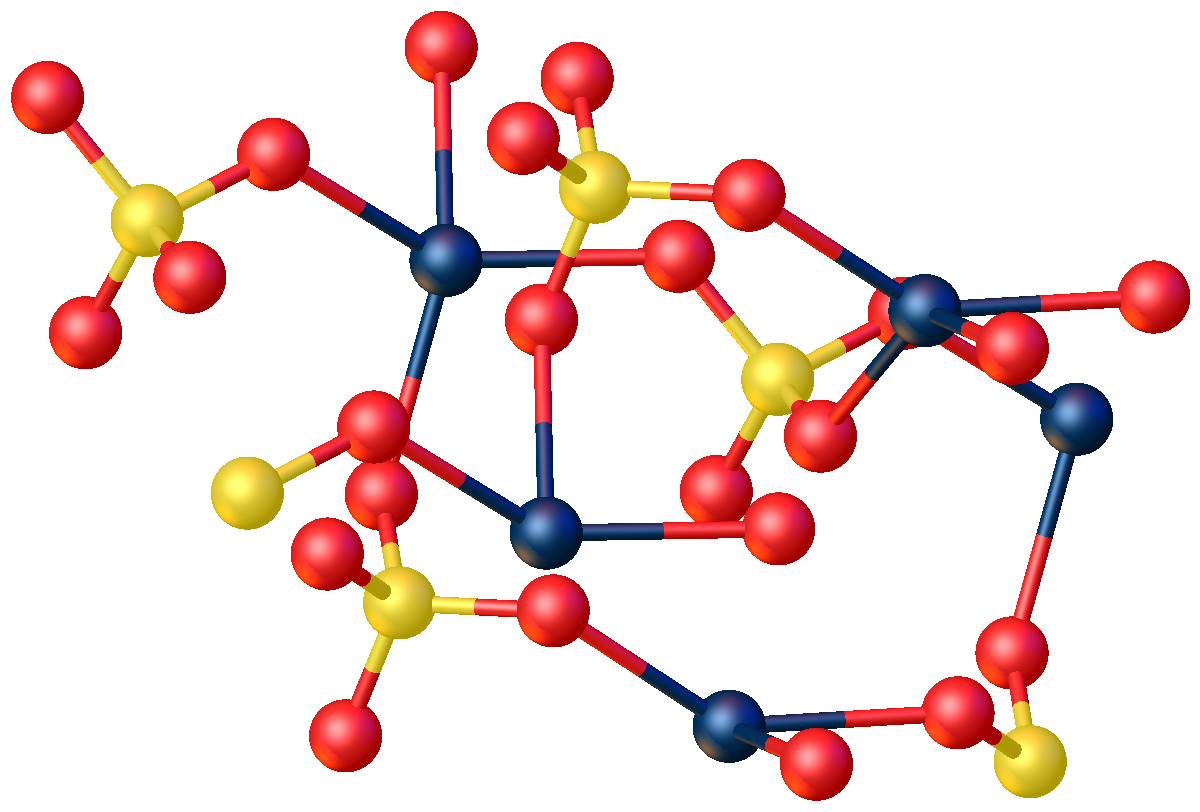
Mercury(II) Sulfate
Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound Hg S O4. It is an odorless solid that forms white granules or crystalline powder. In water, it separates into an insoluble sulfate with a yellow color and sulfuric acid. Structure The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å. In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å. History In 1932, the Japanese chemical company Chisso Corporation began using mercury sulfate as the catalyst for the production of acetaldehyde from acetylene and water. Though it was unknown at the time, methylmercury is formed as side product of this reaction. Exposure and consumption of the mercury waste products, including methylmercury, that were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
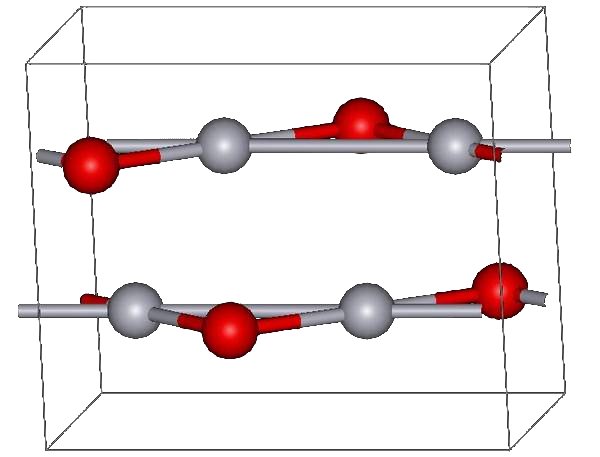
Mercury(II) Oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found. History An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' In 1774, Joseph Priestley discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen (rather, Priestley called it " dephlogisticated air," as that was the paradigm that he was working under at the time). Synthesis The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2. The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali. The difference in color is due to particle size; both forms have the same structure consisting of near ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Reagents
Generally speaking, analytic (from el, ἀναλυτικός, ''analytikos'') refers to the "having the ability to analyze" or "division into elements or principles". Analytic or analytical can also have the following meanings: Chemistry * Analytical chemistry, the analysis of material samples to learn their chemical composition and structure * Analytical technique, a method that is used to determine the concentration of a chemical compound or chemical element * Analytical concentration Mathematics * Abstract analytic number theory, the application of ideas and techniques from analytic number theory to other mathematical fields * Analytic combinatorics, a branch of combinatorics that describes combinatorial classes using generating functions * Analytic element method, a numerical method used to solve partial differential equations * Analytic expression or analytic solution, a mathematical expression using well-known operations that lend themselves readily to calculation * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |