|
Dedicator Of Cytokinesis Protein 4
Dedicator of cytokinesis protein 4 (Dock4), is a large (~190 kDa) protein encoded in the human by the ''DOCK4'' gene, involved in intracellular signalling networks. It is a member of the DOCK-B subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G-proteins. Dock4 activates the small G proteins Rac and Rap1. Discovery Dock4 was discovered as a gene product which was disrupted during tumour progression in a murine cancer model-derived osteosarcoma cell line. Subsequent Northern blot analysis revealed high levels of Dock4 expression in skeletal muscle, prostate and ovary as well as lower levels in the heart, placenta and colon. A separate study has reported expression of a Dock4 splice variant ( Dock4-Ex49) in the brain, inner ear and eye. Structure and function Dock4 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
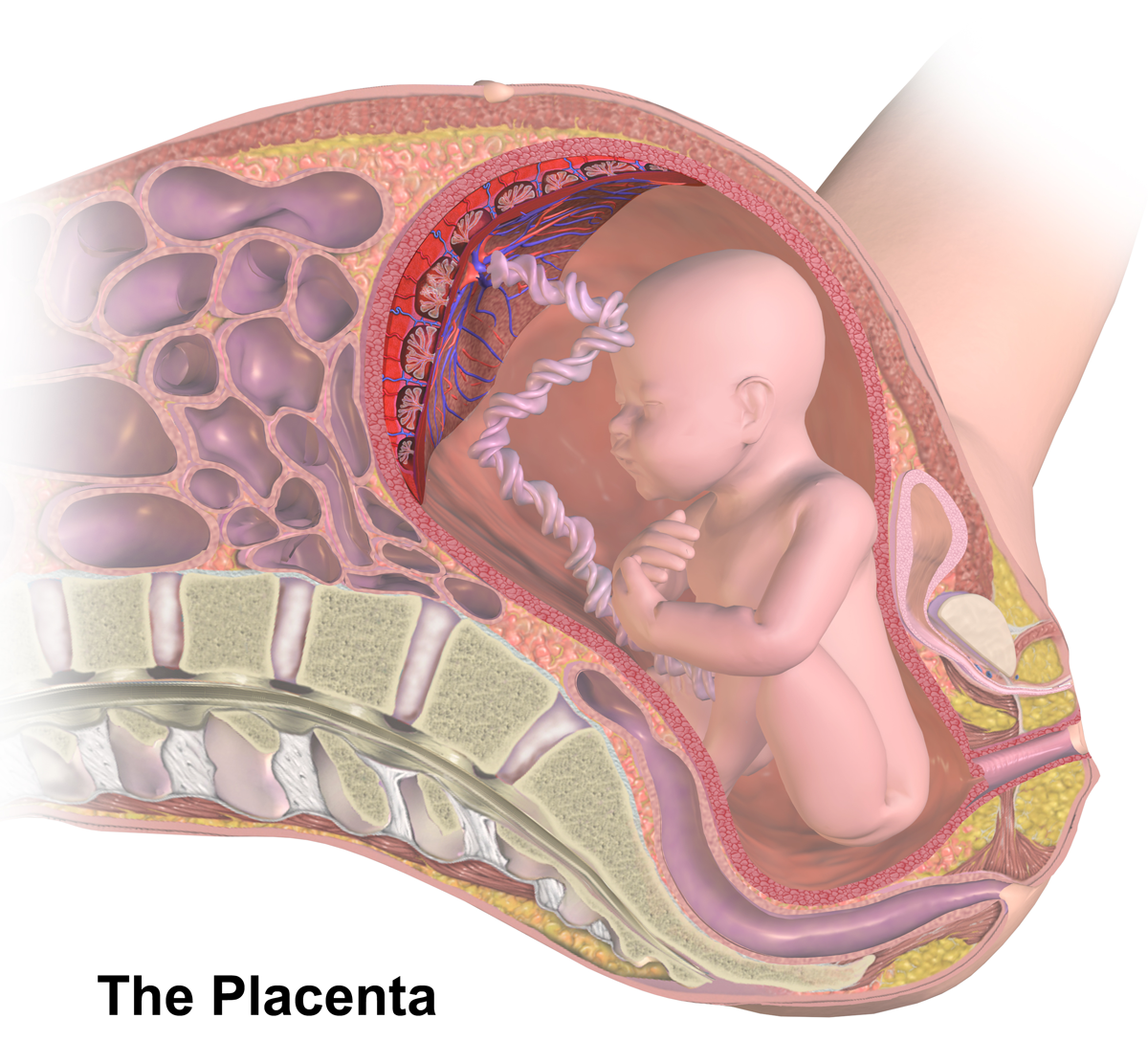
Placenta
The placenta (: placentas or placentae) is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas, and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual ( endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth (sometimes incorrectly referred to as the 'maternal part' of the placenta). Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development. Mammalian placentas probably first evolved abou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have to be associated with a sequence motif; it can be represented by different and completely unrelated sequences in different proteins or RNA. In nucleic acids Depending upon the sequence and other conditions, nucleic acids can form a variety of structural motifs which is thought to have biological significance. ;Stem-loop: Stem-loop intramolecular base pairing is a pattern that can occur in single-stranded DNA or, more commonly, in RNA. The structure is also known as a hairpin or hairpin loop. It occurs when two regions of the same strand, usually complementary in nucleotide sequence when read in opposite directions, base-pair to form a double helix that ends in an unpaired loop. The resulting structure is a key building block of many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dock3
Dedicator of cytokinesis protein 3 (Dock3), also known as MOCA (modifier of cell adhesion) and PBP (presenilin-binding protein), is a large (~180 kDa) protein encoded in the human by the ''DOCK3'' gene, involved in intracellular signalling networks. It is a member of the DOCK-B subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G-proteins. Dock3 specifically activates the small G protein Rac. Discovery Dock3 was originally discovered in a screen for proteins that bind presenilin (a transmembrane protein which is mutated in early onset Alzheimer's disease). Dock3 is specifically expressed in neurons (primarily in the cerebral cortex and hippocampus). Structure and function Dock3 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G proteins are bound to Guanosine diphosphate (GDP) and their activation requires the dissociation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dock2
Dedicator of cytokinesis protein 2 (Dock2) is a protein encoded in the human by the ''DOCK2'' gene. Dock2 is a large (~180 kDa) protein involved in intracellular signalling networks. It is a member of the DOCK-A subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G-proteins. Dock2 specifically activates isoforms of the small G protein Rac. Discovery Dock2 was first characterised as one of a number of proteins which shared high sequence similarity with the previously described protein Dock180, the archetypal member of the DOCK family. Whereas Dock180 expression is near ubiquitous in mammals, Dock2 appears to be expressed specifically in leukocytes and is considered to be the principal DOCK family member in these cells. Structure and function Dock2 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G proteins are bound to Guan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Alignment
In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural biology, structural, or evolutionary relationships between the sequences. Aligned sequences of nucleotide or amino acid residues are typically represented as rows within a matrix (mathematics), matrix. Gaps are inserted between the Residue (chemistry), residues so that identical or similar characters are aligned in successive columns. Sequence alignments are also used for non-biological sequences such as calculating the Edit distance, distance cost between strings in a natural language, or to display financial data. Interpretation If two sequences in an alignment share a common ancestor, mismatches can be interpreted as point mutations and gaps as indels (that is, insertion or deletion mutations) introduced in one or both lineages in the time since they diverged from one another. In sequence ali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dock180
Dedicator of cytokinesis protein 1 (Dock1), also (DOCK180), is a large (~180 kDa) protein encoded in the human by the ''DOCK1'' gene, involved in intracellular signalling networks. It is the mammalian ortholog of the ''C. elegans'' protein CED-5 and belongs to the DOCK family of guanine nucleotide exchange factors (GEFs). Discovery DOCK180 was identified, using a far-western blotting approach, as a binding partner of the adaptor protein Crk that was able to induce morphological changes in 3T3 fibroblasts. Subsequently it was reported that DOCK180 was able to activate the small GTP-binding protein (G protein) Rac1 and this was later shown to happen via its ability to act as a GEF. Structure and function DOCK180 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G proteins are bound to Guanosine diphosphate (GDP) and their activation requires the dissociation of GDP and binding of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Diphosphate
Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine. GDP is the product of GTP dephosphorylation by GTPases, e.g., the G-proteins that are involved in signal transduction. GDP is converted into GTP with the help of pyruvate kinase and phosphoenolpyruvate. GDP and GTP Hydrolysis of GTP into GDP The hydrolysis of GTP to GDP is facilitated by GTPase enzymes, which utilize a conserved active site motif known as the GTPase-activating protein (GAP). Initially, a water molecule is coordinated by the active site residues of the GTPase enzyme. The water molecule attacks the γ-phosphate of GTP, leading to the formation of a pentavalent transition state. This transition state is stabilized by interactions with the active site residues, including conserved catalytic residues. As a result, the γ-phosphat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inner Ear
The inner ear (internal ear, auris interna) is the innermost part of the vertebrate ear. In vertebrates, the inner ear is mainly responsible for sound detection and balance. In mammals, it consists of the bony labyrinth, a hollow cavity in the temporal bone of the skull with a system of passages comprising two main functional parts: * The cochlea, dedicated to hearing; converting sound pressure patterns from the outer ear into electrochemical impulses which are passed on to the brain via the auditory nerve. * The vestibular system, dedicated to balance (ability), balance. The inner ear is found in all vertebrates, with substantial variations in form and function. The inner ear is innervated by the eighth cranial nerve in all vertebrates. Structure The labyrinth can be divided by layer or by region. Bony and membranous labyrinths The bony labyrinth, or osseous labyrinth, is the network of passages with bony walls lined with periosteum. The three major parts of the bony labyrin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |