|
Data Monitoring Committee
A data monitoring committee (DMC) – sometimes called a data and safety monitoring board (DSMB) – is an independent group of experts who monitor patient safety and treatment efficacy data while a clinical trial is ongoing. Need for a DMC Many randomized clinical trials are double-blind – no one involved with the trial knows what treatment is to be given to each trial participant. Blinding includes the participant, their doctor, and even the study personnel at the company or organization sponsoring the trial. Blinding is breached and true assignments disclosed only after the trial database is finalized and locked against edits (i.e., until it is read-only for statistical analysis). On rare occasions a single participant's treating physician may be unblinded to the participant's study treatment assignment if that information is critical to the participant's immediate health care. Clinical trials may test an unknown procedure or may continue for years, and there is justifiable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Vaccine Announcement - 51159265899
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020, the disease spread worldwide, resulting in the COVID-19 pandemic. The symptoms of COVID‑19 can vary but often include fever, fatigue, cough, breathing difficulties, loss of smell, and loss of taste. Symptoms may begin one to fourteen days after exposure to the virus. At least a third of people who are infected do not develop noticeable symptoms. Of those who develop symptoms noticeable enough to be classified as patients, most (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging), and 5% develop critical symptoms (respiratory failure, shock, or multiorgan dysfunction). Older people have a higher risk of developing severe symptoms. Some complications result in death. Some people continue to experience a range of effects (long COVID) for months or yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
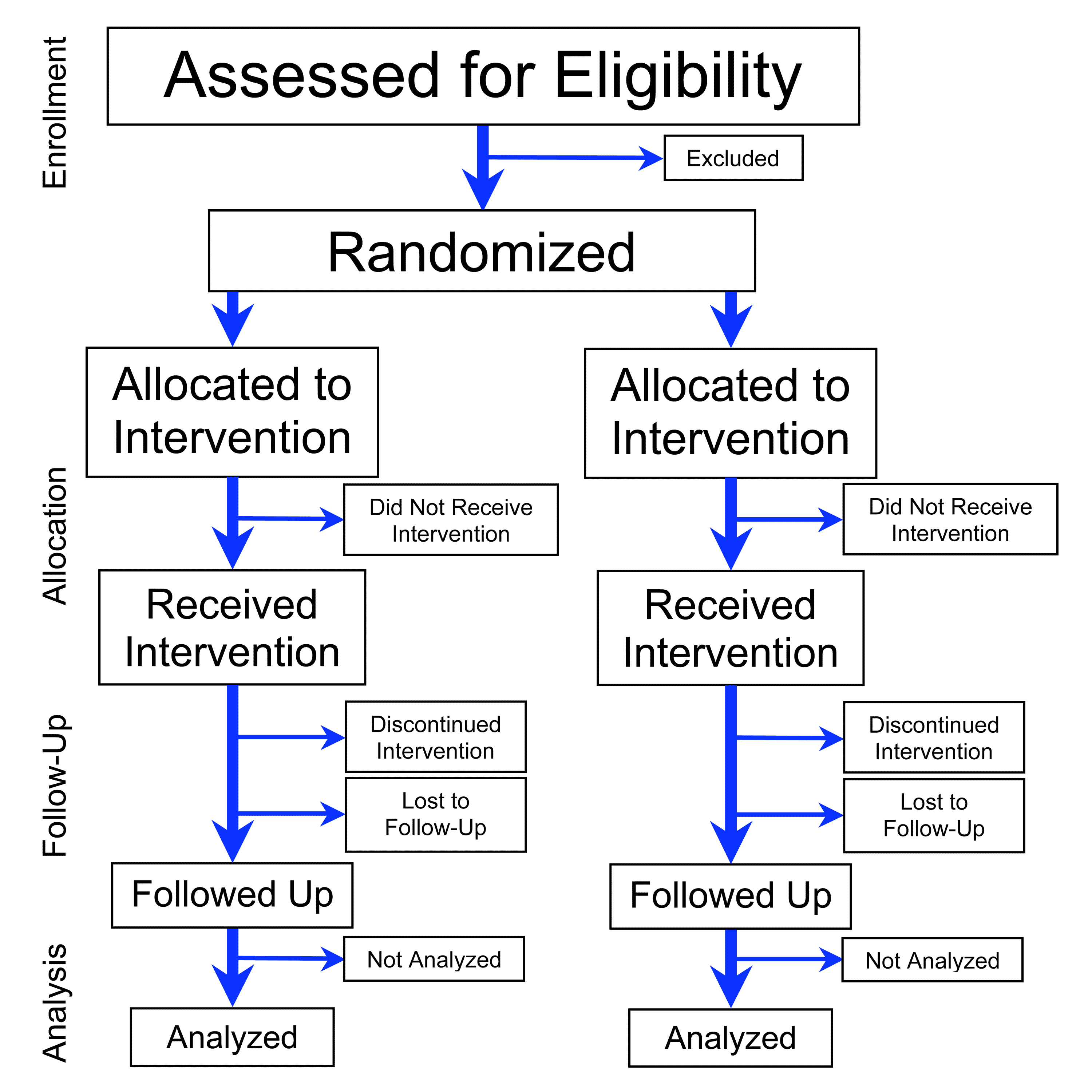
Randomized Clinical Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing standard of care; these are then termed the 'experi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Blind
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints. During the course of an experiment, a participant becomes unblinded if they deduce or otherwise obtain information that has been masked to them. For example, a patient who experiences a side effect may correc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistician
A statistician is a person who works with Theory, theoretical or applied statistics. The profession exists in both the private sector, private and public sectors. It is common to combine statistical knowledge with expertise in other subjects, and statisticians may work as employees or as statistical consultants. Overview According to the United States Bureau of Labor Statistics, as of 2014, 26,970 jobs were classified as ''statistician'' in the United States. Of these people, approximately 30 percent worked for governments (federal, state, or local). As of October 2021, the median pay for statisticians in the United States was $92,270. Additionally, there is a substantial number of people who use statistics and data analysis in their work but have job titles other than ''statistician'', such as Actuary, actuaries, Applied mathematics, applied mathematicians, economists, data scientists, data analysts (predictive analytics), financial analysts, psychometricians, sociologists, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respect For Persons
Respect for persons is the concept that all people deserve the right to fully exercise their autonomy. Showing respect for persons is a system for interaction in which one entity ensures that another has agency to be able to make a choice. This concept is usually discussed in the context of research ethics. It is one of the three basic principles of research ethics stated in the Belmont Report issued by the Office of Human Subject Research; it comprises two essential moral requirements: to recognize the right for autonomy and to protect individuals who are disadvantaged to the extent that they cannot practice this right. An autonomous person is defined as an individual who is capable of self-legislation and is able to make judgments and actions based on their particular set of values, preferences, and beliefs. Respecting a person's autonomy thus involves considering their choices and decisions without deliberate obstruction. It also requires that subjects be treated in a non-deg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Event
In pharmaceuticals, an adverse event (AE) is any unexpected or harmful medical occurrence that happens to a patient during medical treatment or a clinical trial. Unlike direct side effects, an adverse event does not necessarily mean the medication directly caused the problem. These events can include any unfavorable symptoms, signs, or medical conditions that appear during medical treatment, regardless of whether they are definitively linked to the specific medication being studied. AEs in patients participating in clinical trials must be reported to the study sponsor and if required could be reported to the local ethics committee. Adverse events categorized as "serious" (results in death, illness requiring hospitalization, events deemed life-threatening, results in persistent or significant incapacity, a congenital anomaly or medically important condition) must be reported to the regulatory authorities immediately, whereas non-serious adverse events are merely documented in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial Protocol
In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other laboratories. Additionally, and by extension, protocols have the advantage of facilitating the assessment of experimental results through peer review. In addition to detailed procedures, equipment, and instruments, protocols will also contain study objectives, reasoning for experimental design, reasoning for chosen sample sizes, safety precautions, and how results were calculated and reported, including statistical analysis and any rules for predefining and documenting excluded data to avoid bias. Similarly, a protocol may refer to the procedural methods of health organizations, commercial laboratories, manufacturing plants, etc. to ensure their activities (e.g., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
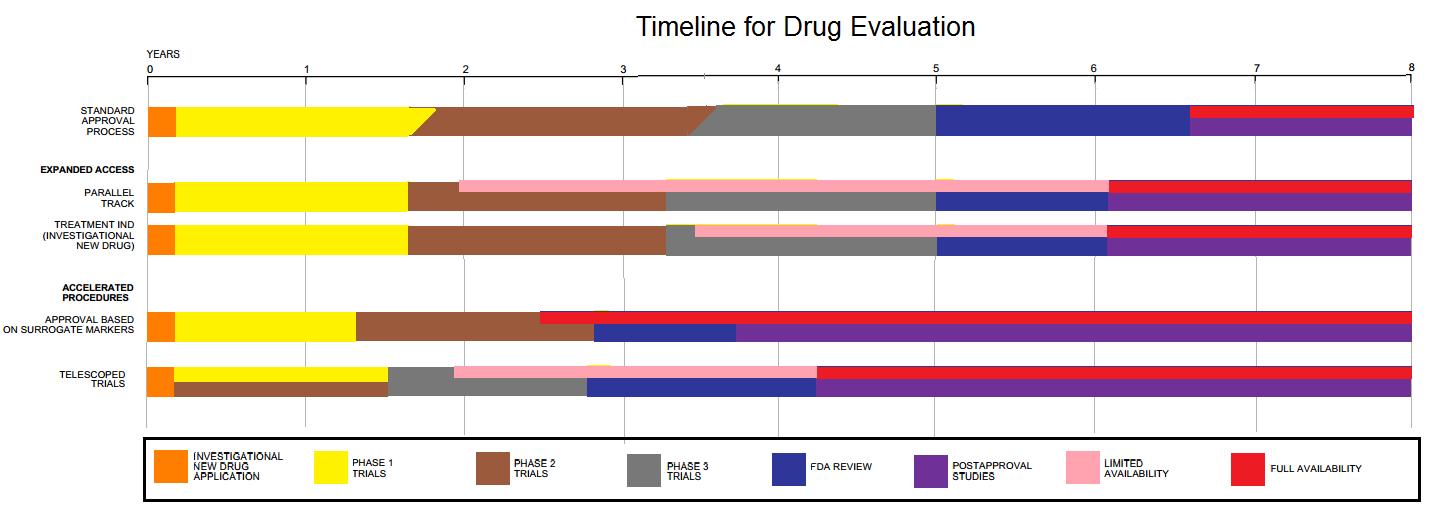
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. These h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EudraVigilance
EudraVigilance (European Union Drug Regulating Authorities Pharmacovigilance) is the European data processing network and management system for reporting and evaluation of suspected Adverse event, adverse reactions to medicines or devices which have received marketing authorisation or are actively being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network. The European EudraVigilance system deals with the: * Electronic exchange of Individual Case Safety Reports (ICSR, based on the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH E2B specifications): ** EudraVigilance Clinical Trial Module (EVCTM) for reporting Suspected Unexpected Serious Adverse Reactions (Serious adverse event, SUSARs). ** EudraVigilance Post-Authorisation Module (EVPM) for post-authorisation ICSRs. * Early det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institutional Review Board
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee at an institution that applies research ethics by reviewing the methods proposed for research involving human subjects, to ensure that the projects are ethical. The main goal of IRB reviews is to ensure that study participants are not harmed (or that harms are minimal and outweighed by research benefits). Such boards are formally designated to approve (or reject), monitor, and review biomedical and behavioral research involving humans, and they are legally required in some countries under certain specified circumstances. Most countries use some form of IRB to safeguard ethical conduct of research so that it complies with national and international norms, regulations or codes. The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of people participating in a research stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monitoring In Clinical Trials
Clinical monitoring is the oversight and administrative efforts that monitor a participant's health and efficacy of the treatment during a clinical trial. Both independent and government-run grant-funding agencies, such as the National Institutes of Health (NIH) and the World Health Organization (WHO), require data and safety monitoring protocols for Phase I and II clinical trials conforming to their standards. Safety monitoring Safety monitoring of a clinical trial is conducted by an independent physician with relevant expertise. This is accomplished by review of adverse event, immediately after they occur, with timely follow-up through resolution. Responsibility for data and safety monitoring depends on the phase of the study and may be conducted by sponsor or Contract research organization (CRO) staff or contractor, and/or by the Principal clinical investigator/project manager conducting the study. Regardless of the method used, monitoring must be performed on a regular basis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |