|
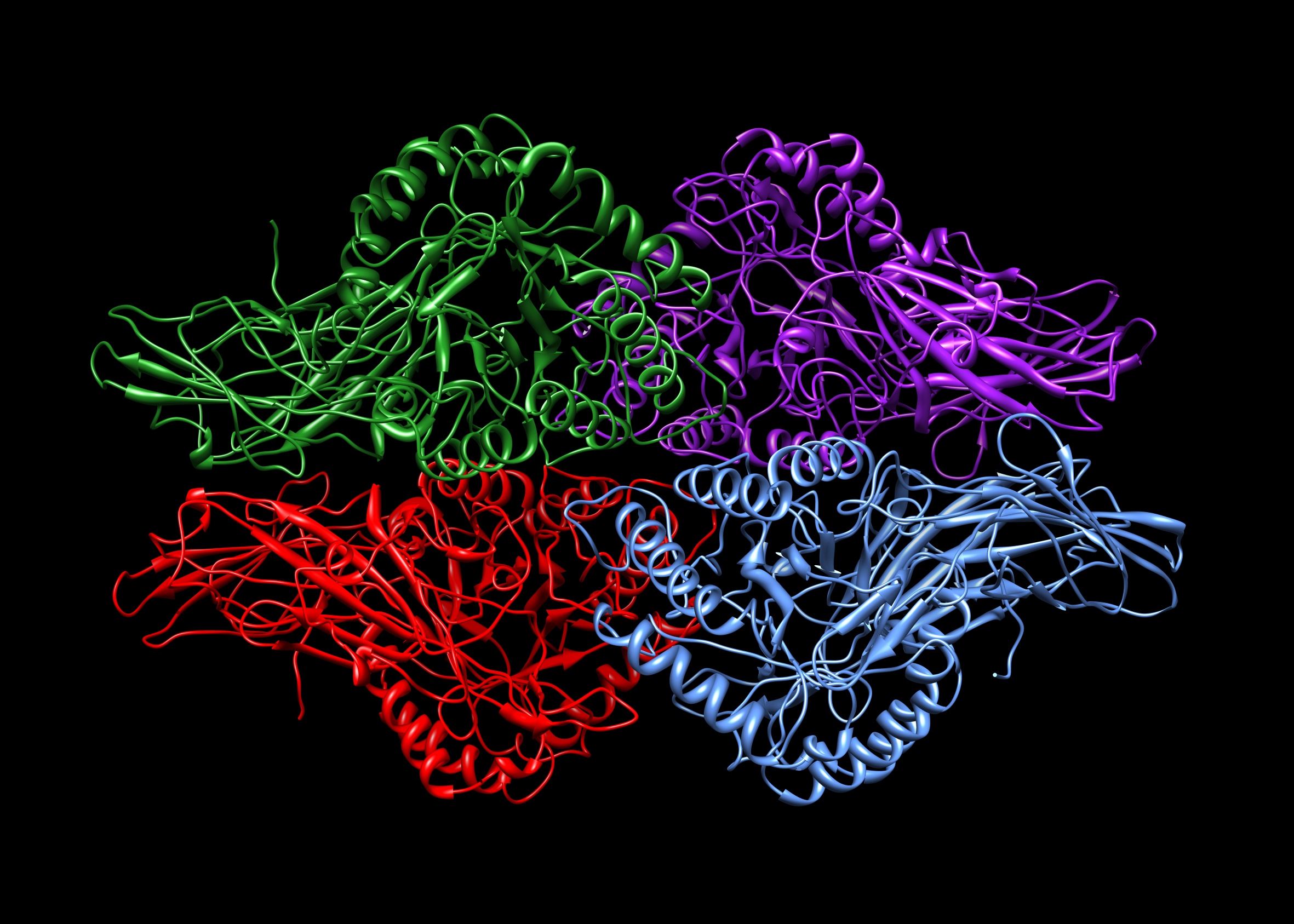
Tetramers (chemistry)
A tetramer () (''wikt:tetra-, tetra-'', "four" + ''wikt:-mer, -mer'', "parts") is an oligomer formed from four monomers or Protein subunit, subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while Antibody, immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the Allosteric regulation, allosteric binding of oxygen in he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carex Folliculata
''Carex folliculata'', the northern long sedge, is a species of flowering plant in the family Cyperaceae. It is native to eastern Canada (and Saint Pierre and Miquelon Saint Pierre and Miquelon ( ), officially the Territorial Collectivity of Saint Pierre and Miquelon (), is a self-governing territorial overseas collectivity of France in the northwestern Atlantic Ocean, located near the Canada, Canadian prov ...), and the eastern United States (but in the southeast it is confined to the Appalachians). A clumping perennial reaching but usually shorter, it has broad, yellowish-green leaves. An obligate wetland species, it is found in a wide variety of wet habitats from sea level up to , and can handle acidic, neutral, and basic conditions. References folliculata Flora of Eastern Canada Flora of Wisconsin Flora of the Northeastern United States Flora of the Southeastern United States Taxa named by Carl Linnaeus Plants described in 1753 {{Carex-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramerium
''Tetramerium'' is a genus of plants belonging to the family Acanthaceae. It is found in the Americas, ranging from the southwestern United States to Bolivia, especially in tropical dry forests. Christian Gottfried Daniel Nees von Esenbeck first described the genus in 1846 after collecting two species (''T. polystachyum'' and ''T. nervosum'') on the journey of . Species 30 species are accepted. * '' Tetramerium abditum'' * '' Tetramerium aureum'' * '' Tetramerium butterwickianum'' * ''Tetramerium carranzae'' * '' Tetramerium crenatum'' * '' Tetramerium denudatum'' * '' Tetramerium diffusum'' * '' Tetramerium emilyanum'' * '' Tetramerium fruticosum'' * '' Tetramerium glandulosum'' * '' Tetramerium glutinosum'' * ''Tetramerium guerrerense'' * '' Tetramerium langlassei'' * '' Tetramerium mcvaughii'' * '' Tetramerium nemorum'' * ''Tetramerium nervosum'' – hairy fornwort - cultivated for xeriscapes * ''Tetramerium oaxacanum'' * ''Tetramerium obovatum'' * ''Tetrame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrameric Protein
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Chemistry
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. Materials can be categorized into three different regimes, namely bulk, nanoparticles and nanoclusters. Bulk metals are electrical conductors and good optical reflectors and metal nanoparticles display intense colors due to surface plasmon resonance. However, when the size of metal nanoclusters is further reduced to form a nanocluster, the band structure becomes discontinuous and breaks down into discrete energy levels, somewhat similar to the energy levels of molecules. This gives nanoclusters similar q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the protein's activity, either enhancing or inhibiting its function. In contrast, substances that bind directly to an enzyme's active site or the binding site of the endogenous ligand of a receptor are called orthosteric regulators or modulators. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avidin
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be ''K''D ≈ 10−15 M, making it one of the strongest known non-covalent bonds. In its tetrameric form, avidin is estimated to be 66–69 k Da in size. 10% of the molecular weight is contributed by carbohydrate, composed of four to five mannose and three N-acetylglucosamine residues The carbohydrate moieties of avidin contain at least three unique oligosaccharide structural types that are similar in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid. Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing tetrahydrothiophene ring fused to a ureido group. A C5-carboxylic acid side chain is appended to the former ring. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12to 20grams of hemoglobin in every 100mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and a globulin. In mammals, hemoglobin makes up about 96% of a red blood cell's dry matter, dry weight (excluding water), and around 35% of the total weight (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL of O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone. The mammalian hemoglobin molecule can bind and transport up to four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotetramer
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |