|
Surface Finishing
Surface finishing is a broad range of industrial processes that alter the surface of a manufactured item to achieve a certain property. Finishing processes may be employed to: improve product appearance, adhesion or wettability, solderability, corrosion resistance, tarnish resistance, the chemical resistance, the wear resistance, hardness, modify electrical conductivity, remove burrs and other surface flaws, and control the surface friction.. In limited cases some of these techniques can be used to restore original dimensions to salvage or repair an item. An unfinished surface is often called '' mill finish''. These processes can improve the durability, performance and even the appearance of the surface being finished. Surface finishing is often one of the final steps taken when working metal and is essential for guaranteeing that metal components meet the requirements of the necessary finish. Surface finishing processes can be categorized by how they affect the workpiece: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohrer Hartmetall Mit Innerer Kühlschmierstoffzufuhr
Bohrer is a German language, German occupational surname literally meaning "one who bores/drills." However, the word “Bohrer” (short for "Bohrmaschine") is in German used also colloquially to refer to a drill. Notable people with the surname include: *Brian Bohrer (born 1960), American pastor and author *Corinne Bohrer (born 1958), American movie and television actress *Doris Bohrer (1923–2016), American intelligence operative *Florence Fifer Bohrer (1877–1960), American politician *Florin Berenguer Bohrer (born 1989), French footballer *Karl Heinz Bohrer (1932–2021), German literary scholar and essayist *Thomas Bohrer (born 1963), American rower References {{surname, Bohrer German-language surnames German occupational surnames ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
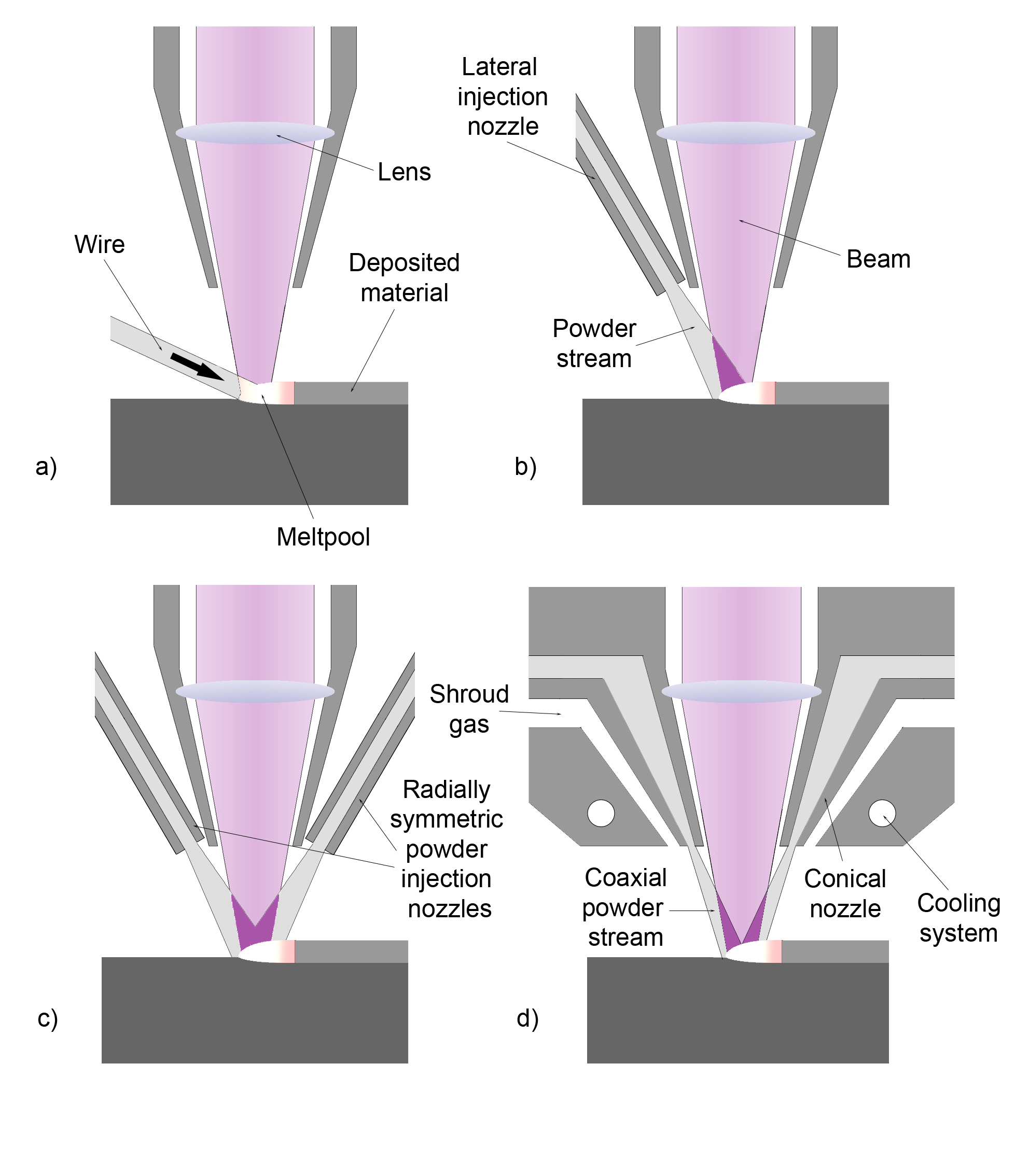
Cladding (metalworking)
Cladding is the bonding together of dissimilar metals. It is different from fusion welding or adhesive, gluing as a method to fasten the metals together. Cladding is often achieved by extrude, extruding two metals through a die (manufacturing), die as well as machine press, pressing or cold rolling, rolling sheets together under high pressure. The United States Mint uses cladding to manufacture coins from different metals. This allows a cheaper metal to be used as a filler. For example, dimes and quarters struck since 1965 have cores made from pure copper, with a clad layer consisting of 75% copper and 25% nickel added during production. Half dollars struck from 1965 to 1969 for circulation and in 1970 for collectors also incorporated cladding, albeit in the case of those coins, the core was a mixture of 20.9% silver and 79.1% copper, and its clad layer was 80% silver and 20% copper. Half dollars struck since 1971 are produced identically to the dimes and quarters. Laser cladding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anodizing
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called ''anodizing'' because the part to be treated forms the anode electrode of an electrolytic cell. Anodizing increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects. Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium, and tantalum. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline micro-electrolytic condit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conversion Coating
A conversion coating is a chemical or electro-chemical treatment applied to manufactured parts that superficially converts the material into a thin adhering coating of an insoluble compound. These coatings are commonly applied to protect the part against corrosion, to improve the adherence of other coatings, for lubrication, or for aesthetic purposes. Types The most common conversion coating processes for metal parts with industrial use include * Chromate (aluminum, steel) * Phosphate (steel) * Bluing (steel) * Black oxide (steel) * Anodizing (aluminum) * Stannate (magnesium) * Molybdate (zinc, zinc-nickel) * Zirconate (steel, aluminum, magnesium, galvanized steel). * Titanate (steel, aluminum, magnesium). * Plasma electrolysis (aluminum, magnesium, titanium) Non-metallic substrates Conversion coatings have been studied for non-metallic substrates, such as for protection of fluorozirconate glasses used in optoelectronics. Regulations US military specifications for co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually an oxide (termed the "native oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paint
Paint is a material or mixture that, when applied to a solid material and allowed to dry, adds a film-like layer. As art, this is used to create an image or images known as a painting. Paint can be made in many colors and types. Most paints are either oil-based or water-based, and each has distinct characteristics. Primitive forms of paint were used tens of thousands of years ago in cave paintings. Clean-up solvents are also different for water-based paint than oil-based paint. Water-based paints and oil-based paints will cure differently based on the outside ambient temperature of the object being painted (such as a house). History Paint was used in some of the earliest known human artworks. Some cave paintings drawn with red or yellow ochre, hematite, manganese oxide, and charcoal may have been made by early ''Homo sapiens'' as long as 40,000 years ago. Paint may be even older. In 2003 and 2004, South African archeologists reported finds in Blombos Cave of a 100,000-y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knurling
Knurling is a manufacturing process, typically conducted on a lathe, whereby a pattern of straight, angled or crossed lines is rolled into the material. Knurling can also refer to material that has a knurled pattern. Etymology The terms ''knurl'' and ''knurled'' are from an earlier ''knur'' ‘knot in wood’ and the diminutive ''-le'', from Middle English ''knaur'' or ''knarre'' ‘knot in wood; twisted rock; crag’. This descends from Old English ''cnearra'' but the vowel in Middle English may have been influenced by Old Norse ''knǫrr'' ‘merchant ship’ which was known as ''cnearr'' in Old English. The modern ''gnarl'' is a back-formation of ''gnarled'' which itself is first attested in Shakespeare's works and is apparently a variant of ''knurled''. Uses Knurling produces indentations on a part of a workpiece, allowing hands or fingers to get a better grip on the knurled object than would be provided by the original smooth surface. Occasionally, the knurled pattern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glaze (metallurgy)
Compacted oxide layer glaze describes the often shiny, wear-protective layer of oxide formed when two metals (or a metal and ceramic) are slid against each other at high temperature in an oxygen-containing atmosphere. The layer forms on either or both of the surfaces in contact and can protect against wear. Background A not often used definition of ''glaze'' is the highly sintered compacted oxide layer formed due to the sliding of either two metallic surfaces (or sometimes a metal surface and ceramic surface) at high temperatures (normally several hundred degrees Celsius) in oxidizing conditions. The sliding or tribological action generates oxide debris that can be compacted against one or both sliding surfaces and, under the correct conditions of load, sliding speed and oxide chemistry as well as (high) temperature, sinter together to form a 'glaze' layer. The 'glaze' formed in such cases is actually a crystalline oxide, with a very small crystal or grain size having been shown ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilding
Gilding is a decorative technique for applying a very thin coating of gold over solid surfaces such as metal (most common), wood, porcelain, or stone. A gilded object is also described as "gilt". Where metal is gilded, the metal below was traditionally silver in the West, to make silver-gilt (or ''vermeil'') objects, but gilt-bronze is commonly used in China, and also called ormolu if it is Western. Methods of gilding include hand application and gluing, typically of gold leaf, chemical gilding, and electroplating, the last also called gold plating. Parcel-gilt (partial gilt) objects are only gilded over part of their surfaces. This may mean that all of the inside, and none of the outside, of a chalice or similar vessel is gilded, or that patterns or images are made up by using a combination of gilt and ungilted areas. Gilding gives an object a gold appearance at a fraction of the cost of creating a solid gold object. In addition, a solid gold piece would often be too soft or to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanizing
Galvanization ( also spelled galvanisation) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are coated by submerging them in a bath of hot, molten zinc. Protective action The zinc coating, when intact, prevents corrosive substances from reaching the underlying iron. It's main function is to act as a sacrificial anode to prevent the iron from rusting by cathodic protection. Zinc is more reactive than iron, so the zinc coating preferentially oxidizes to zinc carbonate, preventing the iron from corroding, even if there are gaps in the zinc coating. Additional electroplating such as a chromate conversion coating may be applied to provide further surface passivation to the substrate material. History and etymology The process is named after the Italian physician, physicist, biologist and philosopher Luigi Galvani (9 September 1737 – 4 December 1798). The earliest known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution (chemistry), solution of a salt (chemistry), salt whose cation is the metal to be coated, and the anode (positive electrode) is usually either a block of that metal, or of some inert electrical conductor, conductive material. The current is provided by an external power supply. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion (mechanical), abrasion and corrosion, lubrication, lubricity, reflectivity, electrical conductivity, or appearance. It is used to build up thickness on undersized or worn-out parts and to manufacture metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |