|
Ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chemicals. Karl Ernst Claus, a Russian scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named it in honor of Russia. (He used the Latin name '' Ruthenia'', which can have other meanings, but specifically stated that the element was named in honor of his "motherland".) Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemical catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photoma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium Crystals
Ruthenium is a chemical element; it has Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chemicals. Karl Ernst Claus, a Russian scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named it in honor of Russian Empire, Russia. (He used the Latin name ''Ruthenia'', which can have other meanings, but specifically stated that the element was named in honor of his "motherland".) Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemical catalysis, catalyst. A new application of ruthenium is as the cappi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hassium
Hassium is a synthetic element, synthetic chemical element; it has chemical symbol, symbol Hs and atomic number 108. It is highly radioactive: its most stable known isotopes have half-life, half-lives of about ten seconds. One of its isotopes, Hs, has Magic number (physics), magic numbers of protons and neutrons for deformed nuclei, giving it greater stability against spontaneous fission. Hassium is a superheavy element; it has been Synthetic element, produced in a laboratory in very small quantities by Nuclear fusion, fusing heavy nuclei with lighter ones. Natural occurrences of hassium have been hypothesized but never found. In the periodic table, hassium is a transactinide element, a member of period 7 and group 8 element, group 8; it is thus the sixth member of the 6d series of transition metals. Chemistry experiments have confirmed that hassium behaves as the heavier Homologous series, homologue to osmium, reacting readily with oxygen to form a volatile tetroxide. The chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Ernst Claus
Karl Ernst Claus, also known as Karl Klaus or Carl Claus (; 22 January 1796 – 24 March 1864), was a Russian chemist and naturalist of Baltic German origin. Claus was a professor at Kazan State University and a member of the Russian Academy of Sciences. He was primarily known as a chemist and discoverer of the chemical element ruthenium, which he named after his homeland of Russia, but also as one of the first scientists who applied quantitative methods in botany.Клаус, Карл Карлович in Волков В.А. ''et al'' "Выдающиеся химики мира: Биографический справочник" Moscow, Высш. шк., 1991 (in Russian) Early life and education Karl Claus was born in 1796 in |
Group 8 Element
Group 8 is a group (column) of chemical elements in the periodic table. It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). "Group 8" is the modern standard designation for this group, adopted by the IUPAC in 1990. It should not be confused with "group VIIIA" in the CAS system, which is group 18 (current IUPAC), the noble gases. In the older group naming systems, this group was combined with groups 9 and 10 and called group "VIIIB" in the Chemical Abstracts Service (CAS) "U.S. system", or "VIII" in the old IUPAC (pre-1990) "European system" (and in Mendeleev's original table). The elements in this group are all transition metals that lie in the d-block of the periodic table. While groups (columns) of the periodic table are usually named after their lightest member (as in "the oxygen group" for group 16), iron group has historically been used differently; most often, it means a set of adjacent elements on period (row) 4 of the table that includes iron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element. When experimentally measured using X-ray crystallography, it has a density of . Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen Nib (pen)#Nib tipping, nib tipping, electrical contacts, and in other applications that require extreme durability and hardness. Osmium is among the Abundance of elements in Earth's crust, rarest elements in the Earth's crust, making up only 50 parts per trillion (Parts-per notation#Parts-per expressions, ppt). Characteristics Physical properties Osmium is a hard, brittle, blue-gray metal, and the densest stable element—about twice as dense as lead. The density of os ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence (chemistry)
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two bonds, triple bonds to be three, quadruple bonds to be four, quintuple bonds to be five and sextuple bonds to be six. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom. Description The valence is the combining capacity of an atom of a given element, determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum Group
The platinum-group metals (PGMs) are six noble, precious metallic elements clustered together in the periodic table. These elements are all transition metals in the d-block (groups 8, 9, and 10, periods 5 and 6). The six platinum-group metals are ruthenium, rhodium, palladium, osmium, iridium, and platinum. They have similar physical and chemical properties, and tend to occur together in the same mineral deposits. However, they can be further subdivided into the ''iridium-group platinum-group elements'' (IPGEs: Os, Ir, Ru) and the ''palladium-group platinum-group elements'' (PPGEs: Rh, Pt, Pd) based on their behaviour in geological systems. The three elements above the platinum group in the periodic table (iron, nickel and cobalt) are all ferromagnetic; these, together with the lanthanide element gadolinium (at temperatures below 20 °C), are the only known transition metals that display ferromagnetism near room temperature. History Naturally occurring plati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
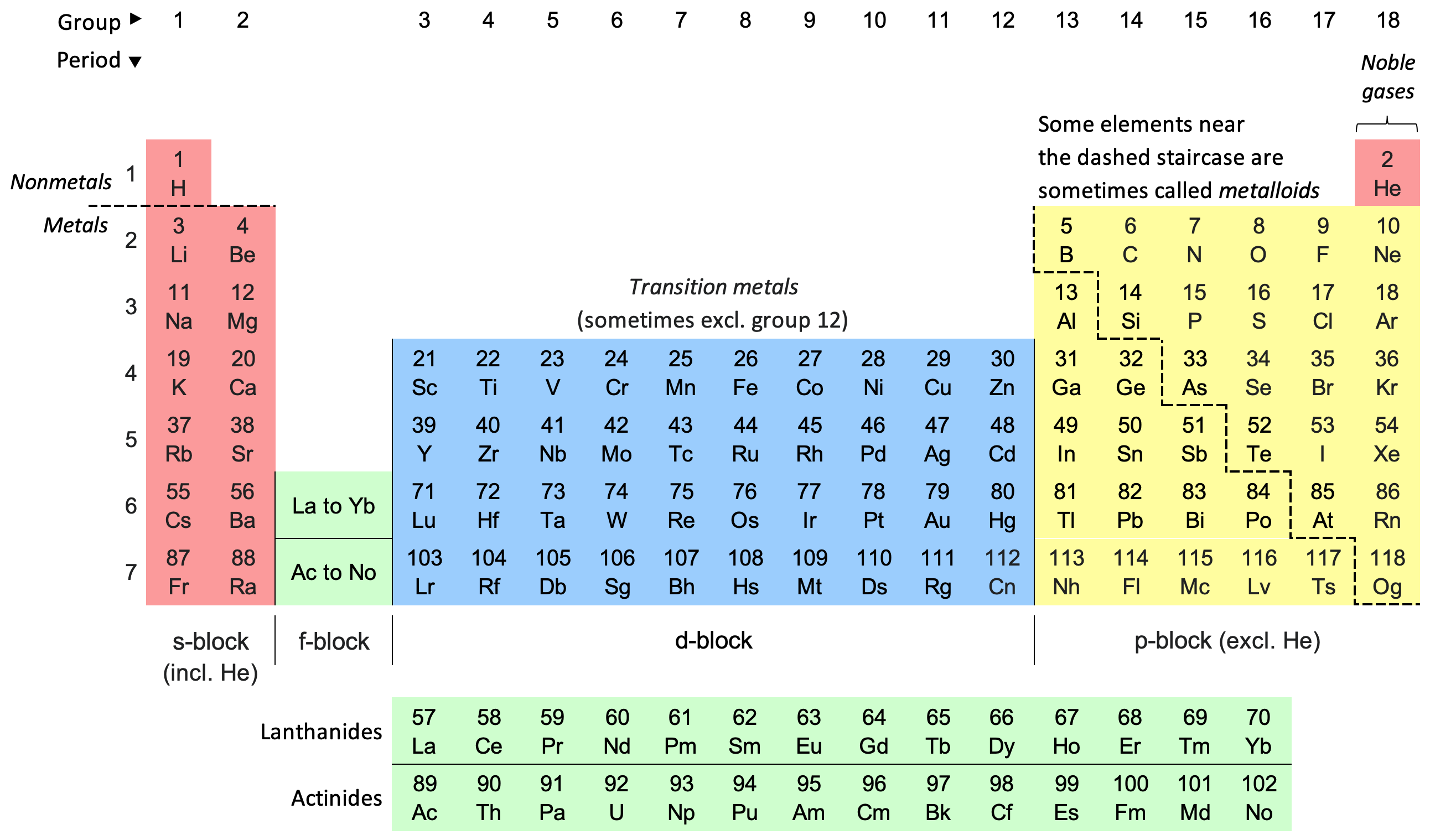
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs hardness rating similar to pure titanium, and it has similar ductility to iron. Niobium oxidizes in Earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite. Its name comes from Greek mythology: Niobe, daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, which makes them difficult to distinguish. English chemist Charles Hatchett reported a new element similar to tantalum in 1801 and named it columbium. In 1809, English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical. German chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenia
''Ruthenia'' is an exonym, originally used in Medieval Latin, as one of several terms for Rus'. Originally, the term ''Rus' land'' referred to a triangular area, which mainly corresponds to the tribe of Polans in Dnieper Ukraine. ''Ruthenia'' was used to refer to the East Slavic and Eastern Orthodox people of the Grand Duchy of Lithuania and the Kingdom of Poland, and later the Polish-Lithuanian Commonwealth and Austria-Hungary, mainly to Ukrainians and sometimes Belarusians, corresponding to the territories of modern Belarus, Ukraine, Eastern Poland and some of western Russia. Historically, in a broader sense, the term was used to refer to all the territories under Kievan dominion (mostly East Slavs). The Kingdom of Galicia and Lodomeria (1772–1918), corresponding to parts of Western Ukraine, was referred to as ''Ruthenia'' and its people as ''Ruthenians''. As a result of a Ukrainian national identity gradually dominating over much of present-day Ukraine in the 19t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |