|
Perylene Dyes
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes. Reactions Like other polycyclic aromatic compounds, perylene is reduced by alkali metals to give a deeply colored radical anion and a dianion. The diglyme solvates of these salts have been characterized by X-ray crystallography. Emission Perylene displays blue fluorescence. It is used as a blue-emitting dopant material in Organic light-emitting diode, OLEDs, either pure or substituted. Perylene can also be used as an organic photoconductor. It has an absorption maximum at 434 nm, and as with all polycyclic aromatic compounds, low water solubility (1.2 x 10−5 mmol/L). Perylene has a molar absorptivity of 38,500 M−1cm−1 at 43 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company has two United States headquarters, in St. Louis and Burlington, MA and has operations in approximately 40 countries. In 2015, the multinational chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. It is headquartered in Burlington, Massachusetts, United States. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Absorptivity
In chemistry, the molar absorption coefficient or molar attenuation coefficient () is a measurement of how strongly a chemical species absorbs, and thereby attenuates, light at a given wavelength. It is an intrinsic property of the species. The SI unit of molar absorption coefficient is the square metre per mole (), but in practice, quantities are usually expressed in terms of −1⋅cm−1 or L⋅mol−1⋅cm−1 (the latter two units are both equal to ). In older literature, the cm2/mol is sometimes used; 1 M−1⋅cm−1 equals 1000 cm2/mol. The molar absorption coefficient is also known as the molar extinction coefficient and molar absorptivity, but the use of these alternative terms has been discouraged by the IUPAC. Beer–Lambert law The absorbance of a material that has only one absorbing species also depends on the pathlength and the concentration of the species, according to the Beer–Lambert law :A = \varepsilon c\ell, where * is the ''molar absorption coef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
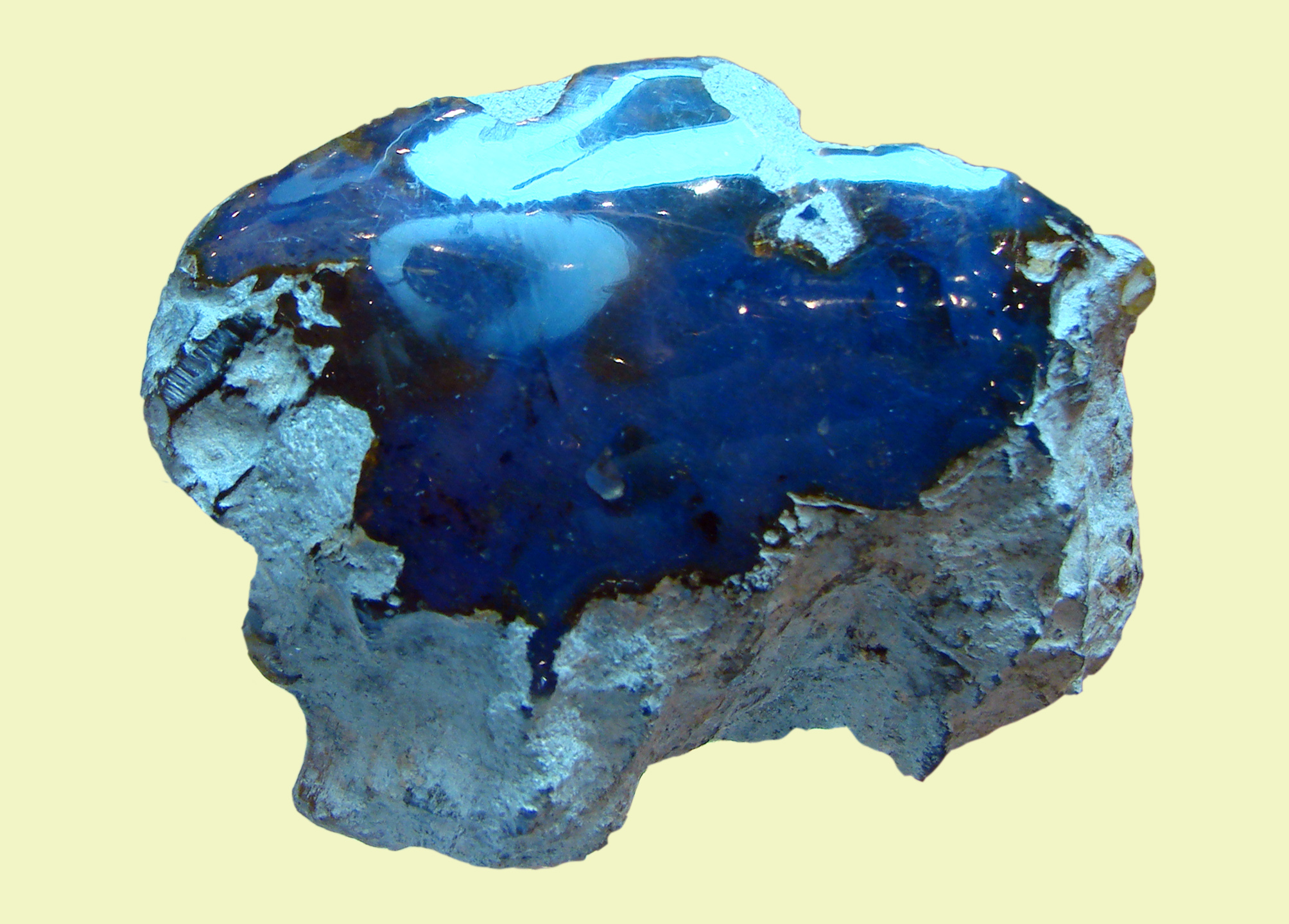
Blue Amber
Blue amber is a rare variety of amber resin that exhibits a blue coloration. Blue amber has been most commonly found in the Dominican Republic—especially in the amber mines around the city of Santiago and, less commonly, in the eastern half of the country. In the modern age, it was discovered at about the same time as Dominican amber. Causes of coloration Vittorio Bellani and Enrico Giulotto at the University of Pavia, Italy, studied several amber specimens by means of optical absorption, fluorescence spectroscopy, and time-resolved fluorescence measurements. The resulting spectral analysis revealed that the emission and excitation spectra were similar in shape to those of diluted solutions of anthracene, perylene, and tetracene, and suggest that the fluorescent hydrocarbon responsible for the blueness is most likely perylene. Despite their findings, the presence of these aromatic hydrocarbons has not been confirmed in samples of blue amber. While all types of amber t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphis (lichen)
''Graphis'' is a genus of lichen-forming fungi in the family Graphidaceae. Historically, ''Graphis'' was used as a broad category for species with colourless, transversely septate ascospores within the Graphidaceae. However, with advances in genetic research, this classification has become more refined. As a result, species previously classified under '' Graphina'' have been re-assigned to ''Allographa'' or ''Graphis''. The species complex around '' Graphis scripta'' has also been recognised, leading to the identification of several new species, many of which may have been previously overlooked. Description Genus ''Graphis'' includes crustose lichens, which have a crust-like appearance that can range from being fully embedded in the substrate to sitting on the surface. The lichen's symbiotic partner, or , is green algae from the genus '' Trentepohlia''. The (fruiting bodies) of ''Graphis'' can also be immersed or superficial. These structures are typically elongated, resembli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laurera
''Laurera'' is a genus of fungi A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ... within the Trypetheliaceae family. Species It was known to have nearly 50 species at one point in time, but most have these have become synonyms for other species, within the '' Astrothelium'' and '' Bathelium'' genera (both still within the Trypetheliaceae family). Leaving; * '' Laurera chrysocarpa'' * '' Laurera sepulta'' References Trypetheliaceae Taxa named by Ludwig Reichenbach {{Dothideomycetes-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lichens
A lichen ( , ) is a hybrid colony (biology), colony of algae or cyanobacteria living symbiotically among hypha, filaments of multiple fungus species, along with yeasts and bacteria embedded in the cortex or "skin", in a mutualism (biology), mutualistic relationship.Introduction to Lichens – An Alliance between Kingdoms . University of California Museum of Paleontology. . Lichens are the lifeform that first brought the term symbiosis (as ''Symbiotismus'') into biological context. Lichens have since been recognized as important actors in nutrient cycling and producers which many higher trophic feeders feed on, such as reindeer, gastropods, nematodes, mites, and springtails. Lichens have properties different from those of their component organisms. They come in man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proceedings Of The Royal Society A
''Proceedings of the Royal Society'' is the main research journal of the Royal Society. The journal began in 1831 and was split into two series in 1905: * Series A: for papers in physical sciences and mathematics. * Series B: for papers in life sciences. Many landmark scientific discoveries are published in the Proceedings, making it one of the most important science journals in history. The journal contains several articles written by prominent scientists such as Paul Dirac, Werner Heisenberg, Ernest Rutherford, Erwin Schrödinger, William Lawrence Bragg, Lord Kelvin, J.J. Thomson, James Clerk Maxwell, Dorothy Hodgkin and Stephen Hawking. In 2004, the Royal Society began '' The Journal of the Royal Society Interface'' for papers at the interface of physical sciences and life sciences. History The journal began in 1831 as a compilation of abstracts of papers in the '' Philosophical Transactions of the Royal Society'', the older Royal Society publication, that began in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation, ppm by mass. As an Aromaticity, aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd (chemist), John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |