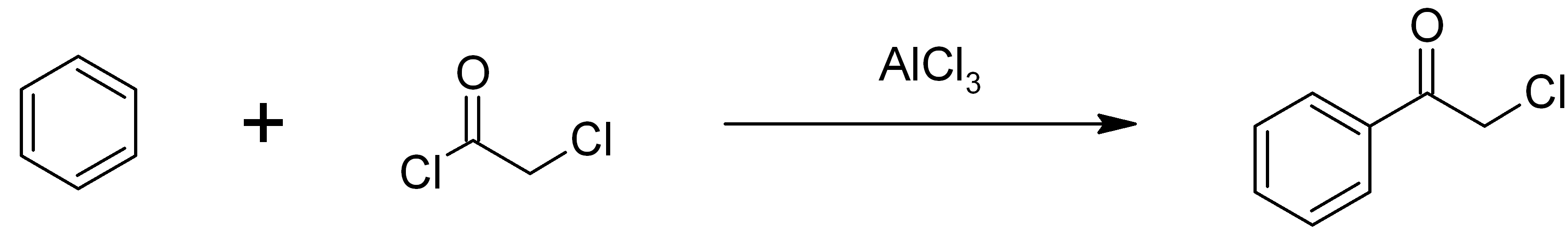|
Oxime Esters
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
General Structure Of Oximes
A general officer is an officer of high rank in the armies, and in some nations' air and space forces, marines or naval infantry. In some usages, the term "general officer" refers to a rank above colonel."general, adj. and n.". OED Online. March 2021. Oxford University Press. https://www.oed.com/view/Entry/77489?rskey=dCKrg4&result=1 (accessed May 11, 2021) The adjective ''general'' had been affixed to officer designations since the late medieval period to indicate relative superiority or an extended jurisdiction. French Revolutionary system Arab system Other variations Other nomenclatures for general officers include the titles and ranks: * Adjutant general * Commandant-general * Inspector general * General-in-chief * General of the Air Force (USAF only) * General of the Armies of the United States (of America), a title created for General John J. Pershing, and subsequently granted posthumously to George Washington and Ulysses S. Grant * (" general admiral" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michigan State University
Michigan State University (Michigan State or MSU) is a public university, public Land-grant university, land-grant research university in East Lansing, Michigan, United States. It was founded in 1855 as the Agricultural College of the State of Michigan, the first of its kind in the country. After the introduction of the Morrill Land-Grant Acts, Morrill Act in 1862, the state designated the college a land-grant institution in 1863, making it the first of the land-grant colleges in the United States. The college became coeducational in 1870. Today, Michigan State has facilities all across the state and over 634,000 alumni. Michigan State is a member of the Association of American Universities and is Carnegie Classification of Institutions of Higher Education, classified among "R1: Doctoral Universities – Very high research activity". The university's campus houses the Facility for Rare Isotope Beams, the W. J. Beal Botanical Garden, the Abrams Planetarium, the Wharton Center f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid, although aged samples appear yellow or even brown. It is a widely used building block in organic synthesis. Preparation and reactions It can be prepared by Dehydration reaction, dehydration of cyanoacetamide. This method is mainly practiced in China where environmental rules are lax. Most commonly malononitrile is produced by the gas-phase reaction of acetonitrile and cyanogen chloride: : About 20,000,000 kg are produced annually (2007). Important outlets include the synthesis of thiamine, the drug triamterene and minoxidil, and the dyes disperse Yellow 90 and disperse Blue 354. Malononitrile is relatively acidic, with a Acid dissociation constant, p''K''a of 11 in water. This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas: Despite its relative obscurity, Malononitrile is very useful in several reactions, the prime example being a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacyl Chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Chloroacetophenone is thermally stable, and is the only tear agent that is distillable at ambient conditions. Preparation Chloroacetophenone was first synthetized by Carl Graebe in 1871 by passing chlorine into boiling acetophenone. Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: : Riot control agent It was investigated, but not used, during the First and Second World Wars (it was used as a "green agent" by the former Japanese mil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propiophenone
Propiophenone (shorthand: benzoylethane or BzEt) is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds. Production Propiophenone can be prepared by Friedel–Crafts reaction of propanoyl chloride and benzene. It is also prepared commercially by ketonization of benzoic acid and propionic acid over calcium acetate and alumina at 450–550 °C: :C6H5CO2H + CH3CH2CO2H → C6H5C(O)CH2CH3 + CO2 + H2O Ludwig Claisen discovered that Alpha and beta carbon, α-methoxystyrene forms this compound when heated for an hour at 300 °C (65% yield). Uses file:Phenmetrazine.svg, 120px, left, Phenmetrazine, derived from propiophenone, is an appetite suppressant. It is an intermediate in the synthesis of the pharmaceuticals phenmetrazine and propoxyphen. Other drugs made from propiophenone include the following: PDM-35, Eprazinone, Methcathinone (leading to ephedrine), Tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Nitrite
The chemical compound ethyl nitrite is an alkyl nitrite with a chemical formula C2H5NO2. It may be prepared from ethanol. : Uses It is used as a reagent with butanone to yield the dimethylglyoxime end product. : Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as ''Witdulsies'', which is sold in pharmacies. It is known as a traditional Afrikaans remedy; the same remedy is apparently made by the Amish in the US. However, FDA has blocked over-the-counter sales of this same remedy, known in the US as ''sweet nitrite'' or ''sweet spirit of nitre'', since 1980. Its use has been associated with fatal methemoglobinemia. Methemoglobinemia Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications ma ... is the primary toxic effect of e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Ethyl Ketone
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts.Wilhelm Neier, Guenter Strehlke "2-Butanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran. Production Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalysed by copper, zinc, or bronze: :CH3CH(OH)CH2CH3 → CH3C(O)CH2CH3 + H2 This is used to produce approximately 700 million kilograms yearly. Other syntheses that have been examined but not implemented include Wacker oxidation of 2-butene and oxidation of isobutylbenzene, which is analogous to the industrial production of ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitrite
Sodium nitrite is an inorganic compound with the chemical formula . It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products. Uses Industrial chemistry The main use of sodium nitrite is for the industrial production of organonitrogen compounds. It is a reagent for conversion of amines into diazo compounds, which are key precursors to many dyes, such as diazo dyes. Nitroso compounds are produced from nitrites. These are used in the rubber industry. It is used in a variety of metallurgical applications, for phosphatizing and detinning. Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. Preparation At large scale, ethyl acetoacetate is industrially produced by treatment of diketene with ethanol. The small scale preparation of ethyl acetoacetate is a classic laboratory procedure. It involves Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol. : Reactions Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 8% of the total. The enol is moderately acidic. Thus ethyl acetoacetate behaves similarly to acetylacetone: : The resulting carbanion undergoes nucleophilic substitution. Ethyl acetoacetate is often used in the acetoacetic ester synthesis, comparable to diethyl malonate in the malonic ester synthesis or the Kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoamyl Nitrite
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group (the amyl in this case) is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867. Uses * Amyl nitrite was historically employed medically to treat heart diseases as well as angina. * Amyl nitrite was sometimes used as an antidote for cyanide poisoning. It was thought to act as an oxidant, to induce the formation of methemoglobin. Methemoglobin in turn can sequester cyanide as cyanomethemoglob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |